While mortality in patients with hypertensive emergency has significantly improved over the past decades, the incidence and complications associated with acute hypertension-mediated organ damage have not followed a similar trend. Hypertensive emergency is characterized by an abrupt surge in blood pressure, mostly occurring in people with pre-existing hypertension to result in acute hypertension-mediated organ damage. Acute hypertension-mediated organ damage commonly affects the cardiovascular system, and present as acute heart failure, myocardial infarction, and less commonly, acute aortic syndrome. Elevated cardiac troponin with or without myocardial infarction is one of the major determinants of outcome in hypertensive emergency.
- hypertensive emergency
- epidemiology
- pathophysiology
- cardiac acute hypertension-mediated organ damage
- myocardial injury
- diagnosis
- classifications
1. Introduction
2. Epidemiology
Although the availability of effective and well-tolerated antihypertensive medications has significantly improved outcomes in patients with hypertensive emergencies, the incidence remains unchanged [13][14][13,14]. An estimated 2–3% of hypertensive patients will develop hypertensive emergency in their lifetime [15][16][15,16]. Data on gender differences in patients with hypertensive emergency have been inconsistent, with some studies showing a predominance of males [7][8][17][18][7,8,17,18], and others showing comparable prevalence in males and females [6][15][19][6,15,19]. Similarly, reports of age distribution compared with patients having acute severe hypertension without acute hypertension-mediated organ damage has been contradictory [9]. Studies report a varying prevalence of cardiac acute hypertension-mediated organ damage, depending on demographics and comorbidities, among others; however, cardiac involvement predominates in most of the studies, with a cumulative prevalence ranging from 3.6 to 91% (Table 1). Reasons for this marked variation in prevalence include: (1) selection bias due to preferential referrals to specialized centers; (2) variation in the exclusion criteria applied; (3) selective use of cardiac troponin assays resulting in underdiagnosis of atypical cases of myocardial infarction; (4) the non-inclusion of patients managed at primary and secondary care levels without referral to tertiary centers where most of the studies were carried out. A recent systematic review reported a composite prevalence of 52% for cardiac involvement in patients with hypertensive emergencies [9]. Epidemiology of the different cardiac acute hypertension-mediated organ damage is further discussed in the section for specific cardiac complications of hypertensive emergency.|
Author, Year, Country |
Design |
AHF (%) |
AMI (%) |
AAS (%) |
Cumulative (%) |
NIMI (%) |
Comments |
|---|---|---|---|---|---|---|---|
|
Fragoulis [6], 2021, Greece |
Prospective |
58 |
22.6 |
2 |
82.6 |
NR |
National cardiac referral centre registry data. Potential for bias towards cardiac complications. |
|
Rubin [18], 2019, France |
Prospective |
31 |
NR |
NR |
31% |
63 |
Excluded myocardial infarction from their cohorts and 63% had elevated troponin while 83% had left ventricular hypertrophy. |
|
Zampaglione [15], 1996, Italy |
Prospective |
36.8 |
12 |
2 |
50.8 |
NR |
Cerebral infarction was the most common acute hypertension-mediated organ damage. However, composite of cardiac complications occurred in 50.8%. |
|
Kim [12], 2022, Korea |
CS |
NR |
40.5 |
NR |
40.5 |
60.4 |
Focused on prognostic role of cardiac troponin in acute severe hypertension. Elevated (occurred in 41.6%) and detectable (occurred in 36.5%) cardiac troponin associated with higher mortality at 3 years. |
|
Guiga [20], 2017, France |
CS |
37.4 |
13.8 |
1.8 |
53 |
NR |
Reported higher mortality in hypertensive emergency than hypertensive urgency (12.5 vs. 1.8%). |
|
Salvetti [8], 2021, Italy 2008 data 2015 data |
Prospective |
34 37.5 |
25 25 |
1 0.5 |
60 63 |
NR NR |
Excluded resuscitated cardiac arrest and patients requiring urgent cardiac catheterization. |
|
Pacheco [7], 2103, Mexico |
Prospective |
25.2 |
59.5 |
6.3 |
91 |
NR |
Their cohorts composed of a high-risk group admitted into coronary care unit. Reported high rate of acute coronary syndrome and acute aortic syndrome. |
|
Martin [17], 2004, Brazil |
Retrospective |
25 |
13 |
0 |
33 |
NR |
Reported unstable angina (5%) separately from myocardial infarction (8%). |
|
Vilela-Martin [21], 2011, Brazil |
CS |
30.7 |
25.1 |
3.5 |
47.2 |
NR |
Reported unstable angina (12.1%) separately from myocardial infarction (13%). |
|
Nkoke [19], 2020, Cameroon |
CS |
44.6 |
3.6 |
0 |
48.2 |
NR |
Myocardial infarction occurred in 3.6% of their cohorts. Low rate of detection of myocardial infarction may be related to lack of facilities including low rates of ECG and cardiac troponin assay. |
|
Acosta [22], 2020, USA |
Retrospective |
NR |
1 |
0 |
1 |
15 |
Assessed acute myocardial injury using serial cardiac troponin assay. Excluded acute coronary syndrome from their cohorts. |
|
Pattanshetty [23], 2012, USA |
Retrospective |
20.5 |
11.7 |
2.3 |
34.5 |
NR |
Obstructive coronary artery disease present in 76.5% of their cohorts with elevated cardiac troponin that had angiogram. |
AAS, acute aortic syndrome; AHF, acute heart failure; AMI, acute myocardial infarction; CS, cross-sectional; NIMI, non-ischemic myocardial injury; NR, not reported; USA, United State of America.
3. Pathophysiology
The exact pathophysiologic mechanisms of hypertensive emergency remain incompletely understood. However, a sudden rise in BP serves as a common denominator underlying the various forms of acute hypertension-mediated organ damage, and most hypertensive emergencies occur in people with pre-existing hypertension [4]. Although triggers for the surge in BP are also not clearly understood, nonadherence to antihypertensive medications, stress, and increased salt intake have been identified as major risk factors [6]. Three intrinsically interwoven processes operating in concert play an important role in the pathophysiology. These include the failure of vascular autoregulation, endothelial dysfunction, and activation of the renin angiotensin aldosterone system (RAAS). The principal function of vascular autoregulation is to ensure uninterrupted blood flow to vital organs during fluctuations in BP and perfusion pressure, and this is accomplished via the appropriate modification of the peripheral vascular resistance (PVR) [24][25][24,25]. Vascular resistance is constantly modified by metabolic, myogenic, and endothelial modulators acting in concert [26]. During increased BP and perfusion pressure, vascular resistance increases to mitigate hyper perfusion-induced organ injury, while in the face of hypotension and reduced perfusion pressure, vasodilation results in reduced vascular resistance to maintain flow to vital organs. In hypertensive emergency, a surge in BP and increased intravascular shear stress results in the disruption of vascular autoregulation and endothelial damage. This causes increased vascular permeability, perivascular oedema, exposure of subendothelial contents to circulating blood, and thrombogenesis [27]. The ensuing microvascular damage and thrombotic occlusion results in hemolysis, hypoperfusion, release of cytokines and proinflammatory molecules, ischemia, and activation of the RAAS [27][28][27,28]. Heightened activation of the RAAS and increased levels of angiotensin II is nearly ubiquitous in patients with hypertensive emergency and correlates with the extent of microvascular damage [28]. Angiotensin II is a potent mediator of vasoconstriction, inflammation, endothelial dysfunction, remodeling, and vascular fibrosis, and stimulates the secretion of aldosterone [29]. In addition to its principal role of volume expansion and BP maintenance, aldosterone causes cardiovascular and renal inflammation, fibrosis, and remodeling [30]. Recent studies demonstrated the expression of mineralocorticoid receptors in endothelial and vascular smooth muscle cells, resulting in aldosterone-induced vascular inflammation, fibrosis, and remodeling, as well as vascular smooth muscle cell hypertrophy and proliferation [31][32][33][31,32,33]. RAAS also exerts stimulatory effects on the cerebral sympathetic nervous system and potentiates the release of norepinephrine [34]. Increased levels of norepinephrine are associated with natriuresis, volume contraction, and the activation of RAAS, thus, establishing a vicious cycle. Fibrinoid necrosis of small muscular arteries and arterioles, characterized by medial smooth muscle cell necrosis and the focal deposition of proteinaceous material occurs in malignant hypertension, a form of hypertensive emergency [35]. This is succeeded by proliferative endarteritis, characterized by intimal thickening, hyperplasia of the intimal fibroblasts, generation of collagen fibers, and atrophy of the media. Fibrinoid necrosis and proliferative endarteritis are considered the histological hallmark (but not pathognomonic) of malignant hypertension, and both may result in impaired perfusion and ischemia [35]. These changes have been demonstrated in various organs including the kidney, brain, intestine, and pancreas [36]. In one proof-of-concept study, the intravenous injection of angiotensin II in an experimental model of hypertension resulted in increased endothelial permeability and necrosis of cardiac myocytes and intramyocardial arterioles, with sparing of the epicardial coronary arteries [37]. The constellation of pathophysiologic events described above does not occur in any preferential order, but rather, evolves concurrently in a variety of sequences with overlaps and widespread involvement of the vascular beds across various organs. The combined effects of autoregulatory failure, endothelial dysfunction and RAAS activation establishes a vicious cycle of BP elevation and progressively worsening acute hypertension-mediated organ damage. A summary of the pathophysiological mechanisms is presented in Figure 1.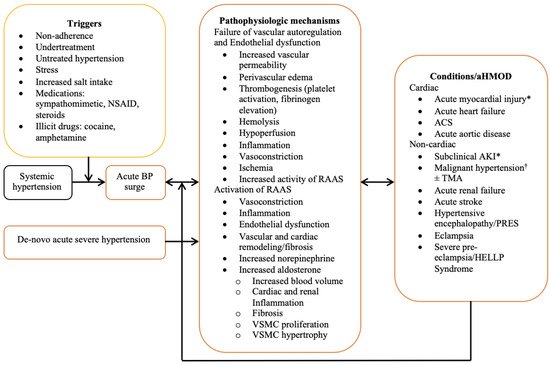
4. Specific Cardiac Complications of Hypertensive Emergency
The different cardiac complications of hypertensive emergency are presented in Table 2.|
Acute hypertension mediated-organ damage |
|
Acute heart failure/acute pulmonary edema * |
|
Acute coronary syndrome * |
|
ST-elevation myocardial infarction |
|
Non-ST-elevation myocardial infarction |
|
Unstable angina |
|
Acute aortic syndrome |
|
Acute aortic dissection * |
|
Intramural hemorrhage/hematoma |
|
Penetrating atherosclerotic aortic ulcer |
|
Aortic aneurysm |
|
Aortic rupture |
|
Sub-clinical cardiac target organ injury § |
|
Acute myocardial injury |
* Commonly reported cardiac complications; § Not included as a complication in guidelines.
