Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Soisungwan Satarug and Version 2 by Conner Chen.
Dietary assessment reports and population surveillance programs show that chronic exposure to low levels of environmental cadmium (Cd) is inevitable for most people, and adversely impacts the health of children and adults.
- cadmium
- dietary exposure
- fecundity
1. Introduction
Cadmium (Cd) is a toxic metal of growing public health concern due to its widespread contamination of staple foods and air, and its exquisite toxicity to multiple organ systems [1][2][3][1,2,3]. Like all other metals, Cd persists indefinitely in the environment because of its nonbiodegradability. Despite its low levels in Earth’s crust and most soils, it can accumulate in vegetation because of its efficient soil-to-plant transference [4][5][6][4,5,6]. Cd is found in high abundance and is associated with the zinc (Zn) ores greenockite and sphalerite; thus, it is a byproduct of the mining, smelting, and refining of Zn ores, and has been used in many industrial processes [5][6][5,6]. Due to the realization of its high toxicity, the worldwide production and industrial uses of Cd have greatly reduced. However, the continued use of Cd-contaminated phosphate fertilizers still adds Cd to the food chain in most parts of the world [7][8][9][7,8,9].
As food crops form the major source of non-workplace Cd exposure in the non-smoking population [10][11][10,11], and because an outbreak of severe Cd poisoning, called “itai-itai” disease, revealed a health threat from the Cd contamination of rice [12][13][12,13], exposure guidelines and toxicity threshold levels of Cd in the human diet were established [14][15][14,15]. In addition, dietary assessment methods such as food-frequency questionnaires, duplicate diet studies and total diet studies (TDSs) have been used to monitor population exposure [10][11][16][10,11,16].
TDS is known also as the “market basket survey” because it involves the collection of samples of foodstuffs from supermarkets and retail stores for the quantitation of various food additives, pesticide residues, contaminants, and nutrients [10][11][10,11]. It is a reasonable method to identify sources as well as to gauge the levels of various contaminants and food additives in the human diet. Various food authority agencies such as the U.S. Food and Drug Administration (FDA), the European Food Safety Agency (EFSA), and the Food Standards of Australia and New Zealand (FSANZ) are tasked with conducting these monitoring programs. Overall, the TDS data indicate that Cd intake varies widely among populations, but the foods that are frequently consumed in large quantities, such as rice, potatoes, wheat, and leafy salad vegetables, are consistently the major sources of Cd [1][2][1,2].
2. Cadmium Tolerable Intake Level and Toxicity Threshold Level
The Joint FAO/WHO Expert Committee on Food Additives and Contaminants (JECFA) considered the kidney to be the critical target of Cd toxicity [14]. By definition, the provisional tolerable weekly intake (PTWI) for a chemical with no known biological function is an estimate of the amount that can be ingested weekly over a lifetime without an appreciable health risk. In 2010, the PTWI for Cd was amended to a tolerable monthly intake (TMI) of 25 μg per kg body weight per month, equivalent to 0.83 μg per kg body weight per day. Similarly, a Cd excretion rate of 5.24 μg/g creatinine was adopted as a nephrotoxicity threshold value [14]. The tolerable intake level derived by JECFA was based on a risk assessment model that considered an increase in the excretion rate of the low-molecular-weight protein β2-microglubulin (β2M) above 300 μg/g creatinine to be a “critical” endpoint. The European Food Safety Authority (EFSA) accepted the same endpoint. However, the EFSA designated a Cd excretion rate of 1 μg/g creatinine as the toxicity threshold with their inclusion of an uncertainty factor (safety margin), where an intake of 0.36 μg/kg body weight per day for 50 years was derived as an acceptable Cd ingestion level or reference dose (RfD) [15][16][15,16]. In theory, a threshold of toxicity is defined as the highest dose that does not produce an adverse effect in the most sensitive organ [17]. In a recent assessment, β2M excretion levels of 100–299, 300–999, and ≥1000 μg/g creatinine were associated with 4.7-, 6.2- and 10.5-fold increases in the risk of an estimated glomerular filtration rate (eGFR) ≤ 60 mL/min/1.73 m2, commensurate with CKD [18]. Thus, a cut-off value for an elevation of β2M excretion above 300 μg/g creatinine does not appear to be an early warning sign of the nephrotoxicity of Cd. The utility of β2M excretion as a toxicity criterion to derive a toxicity threshold level for Cd is questionable. A further discussion on β2M excretion as a marker of tubulopathy is provided in Section 4.4.3. Organs Susceptible to Cadmium Toxicity
3.1. Fate of Cadmium in the Body
As Figure 1 depicts, ingested Cd is absorbed by the intestine and transported via the portal blood system to the liver, where its uptake induces the synthesis of metallothionein (MT) and the formation of CdMT complexes [19]. Later, hepatic CdMT is released into the systemic circulation. The fraction of absorbed Cd not taken up by hepatocytes in the first pass reaches systemic circulation and is taken up by tissues and organs throughout the body, including the kidneys, pancreas [20], ovaries [21] and testes [22].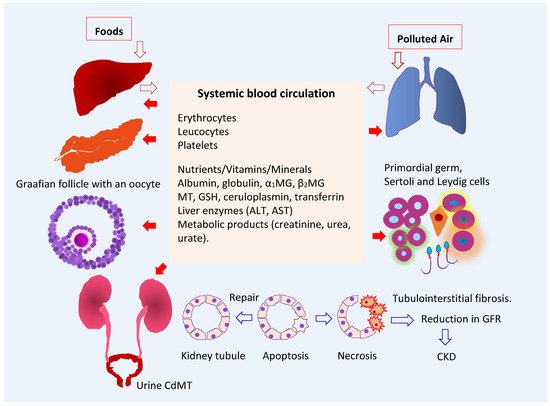
Figure 1. Multiple toxicity targets of cadmium. Ingested Cd is absorbed and transported to liver, where synthesis of MT is induced, and CdMT is formed. The fraction of absorbed Cd not taken up by hepatocytes in the first pass reaches systemic circulation and is taken up and accumulated by cells throughout the body. After glomerular filtration, CdMT is reabsorbed by kidney tubular cells. Other forms of filtered Cd can be reabsorbed by the kidney nephron transporters for iron, zinc, manganese, and calcium. Abbreviations: Cd—cadmium; MT—metallothionein; CdMT—cadmium-metallothionein complex; α1MG—α1-microgloulin; β2MG—β2-microglobulin; GSH—glutathione; ALT—alanine aminotransferase; AST—aspartate aminotransferase; GFR—glomerular filtration rate; CKD—chronic kidney disease.
3.2. Target Organ Toxicity Identified from U.S. NHANES
As discussed in Section 2 above contents, an increase in β2M excretion above 300 μg/g creatinine was used as an endpoint in the health-risk assessment of Cd in the human diet, and urinary Cd excretion levels below 5.24 µg Cd/g creatinine were identified as the body burdens that were not associated with a change in β2M excretion [14]. Consequently, renal tubular dysfunction has become the most frequently reported adverse effect of environmental Cd exposure. However, many population-based studies in many countries and the U.S. general population study known as National Health and Nutrition Examination Survey (NHANES) have provided ample evidence that Cd exposure may impact the functions of many organ systems at Cd excretion levels below 5 µg/g creatinine. NHANES is a cross-sectional study that has provided data on levels of exposure to more than 200 chemicals [26]. Urinary and blood Cd levels were quantified via a standardized methodology that enables the comparison of data across NHANES cycles [26]. The average Cd consumption estimated for the U.S. general population was 4.63 μg/d [27]. This figure was based on 24 h dietary recalls obtained for NHANES 2007–2012 participants aged 2 years and older (n = 12,523), plus the Cd levels of 260 food items in the 2006–2013 market basket surveys [27]. Cereals and bread, leafy vegetables, potatoes, legumes and nuts, stem/root vegetables, and fruits contributed to 34%, 20%, 11%, 7%, and 6% of total intake, respectively. Foods that contain relatively high Cd levels are spaghetti, bread, potatoes, and potato chips which contributed the most to total Cd intake, followed by lettuce, spinach, tomatoes, and beer. Lettuce was a main Cd source for White people and Black people. Tortillas and rice were the main Cd sources for Hispanic Americans and Asians plus other ethnicities [27]. The geometric mean, the 50th, 75th, 90th, and 95th percentile values for urinary Cd levels in the representative U.S. general population were 0.210, 0.208, 0.412, 0.678, and 0.949 µg/g creatinine, and the corresponding values for blood Cd were 0.304, 0.300, 0.500, 1.10, and 1.60 µg/L, respectively [28]. Based on the above figures for dietary exposure and urinary and blood Cd levels, environmental Cd exposure levels in the U.S. could be considered as low. The urinary excretion of Cd and its blood levels associated with adverse effects on the kidneys [29][30][31][32][29,30,31,32], liver [33][34][35][33,34,35], and pancreas [36][37][38][36,37,38] are provided in Table 1.Table 1.
Kidney, liver and pancreas as targets of toxicity to chronic exposure to low-dose cadmium.
| Targets | NHANES Dataset | Adverse Effects and Risk Estimate | References | ||||
|---|---|---|---|---|---|---|---|
| Kidneys | 1999–2006 | Blood Cd levels >1 µg/L were associated with low GFR | a | (OR 1.48) and albuminuria | b | (OR 1.41). The OR for albuminuria was increased to 1.63 in those with urinary Cd ≥ 1 µg/g creatinine plus blood Cd > 1 µg/L. |
Ferraro et al. 2010 [29] |
| Kidneys | 1999–2006 | Blood Cd levels ≥ 0.6 μg/L were associated with low GFR (OR 1.32), albuminuria (OR 1.92) and low GFR plus albuminuria (OR 2.91). | Navas-Acien et al. 2009 [30] | ||||
| Kidneys | 2011–2012 | Blood Cd levels ≥ 0.53 μg/L were associated albuminuria (OR 2.04) and low GFR (OR 2.21). | Lin et al. 2014 [31] |
||||
| Kidneys | 2007–2012 | Blood Cd ≥ 0.61 μg/L were associated with low GFR (OR 1.80) and albuminuria (OR 1.60). | Madrigal et al. 2019 [32] | ||||
| Liver | 1988–1994 | Urinary Cd levels ≥ 0.83 μg/g creatinine were associated with liver inflammation in women (OR 1.26). Urinary Cd ≥ 0.65 μg/g creatinine were associated with liver inflammation (OR 2.21), NAFLD (OR 1.30), and NASH (OR 1.95) in men. |
Hyder et al. 2013 [33] |
||||
| Liver | 1999–2015 | A 10-fold increment of urinary Cd was associated with elevated plasma levels of total bilirubin (OR 1.20), ALT (OR 1.36), and AST (OR 1.31). | Hong et al. 2021 [34] |
||||
| Liver | 1999–2016 | A urinary Cd quartile 4 was associated with elevated plasma ALT (OR 1.40) and AST (OR 1.64). The effect was larger in boys than in girls. | Xu et al. 2022 [35] |
||||
| Pancreas | 1988–1994 | Urinary Cd levels 1–2 μg/g creatinine were associated prediabetes (OR 1.48) and diabetes (OR 1.24). |
Schwartz et al. 2003 [36] | ||||
| Pancreas | 2005–2010 | Urinary Cd levels ≥ 1.4 µg/g creatinine were associated with pre-diabetes in non-smokers. In a fully adjusted model including smokers and non-smokers, urinary Cd levels between 0.7 and 0.9 µg/g creatinine were associated with pre-diabetic risk. | Wallia et al. 2014 [37] |
||||
| Pancreas | 1999–2006 | Urinary Cd levels of 0.198 and 0.365 μg/g creatinine were identified as exposure levels at which the prevalence of type 2 diabetes was smaller than 5% and 10%, respectively. | Shi et al. 2021 [38] |
NHANES—National Health and Nutrition Examination Survey; a low GFR is defined as estimated glomerular filtration rate (eGFR) ≤ 60 mL/min/1.73 m2; b albuminuria is defined as urinary albumin-to-creatinine ratio ≥ 30 mg/g. OR—odds ratio; NAFLD—non-alcoholic fatty liver disease; NASH—non-alcoholic steatohepatitis.
The associations between Cd expsoure and reduced eGFR and albuminuria were consistently observed among participants in NHANES cycles undertaken between 1999 and 2016. A further analysis of these kidney outcomes is provided in Section 4.5. For liver outcomes, the hepatotoxicity of Cd in children appeared to be more pronounced in boys than girls [35]. In adults, urinary Cd levels ≥ 0.6 µg/g creatinine were associated with an increased risk of liver inflammation, NAFLD, and NASH in adults [33]. In Korean population studies, Cd hepatotoxicity was observed at blood Cd levels of 1–2 µg/L [39][40][39,40].
Urinary Cd levels of 1–2 µg/g creatinine were associated with increases in the risks of prediabetes and diabetes among U.S. adults [36][37][36,37]. Cd exposure was associated with an elevated risk of diabetes in a community-based study in Dallas, Texas [41]. A risk analysis using data from 4530 adults enrolled in NHANES 1999–2006, of which 10.3% had diabetes. Urinary Cd levels of 0.198 and 0.365 μg/g creatinine were found to be the benchmark dose (BMD) limit5 and BMDL10 for type 2 diabetes [38]. Thus, urinary Cd levels of 0.198 and 0.365 μg/g creatinine were the body burdens at which the prevalence of type 2 diabetes was likely smaller than 5% and 10%, respectively [38]. In a meta-analysis of pooled data from 42 studies, the risks of prediabetes and diabetes increased linearly with blood and urinary Cd; prediabetes risk reached a plateau at a urinary Cd of 2 µg/g creatinine, and the diabetes risk rose as blood Cd reached 1 µg/L [42]. These urinary Cd and blood Cd levels are also in range with those associated with reduced eGFR and albuminuria in studies conducted in the Torres Straits, Australia [43], Thailand [44], and Taiwan [45].
