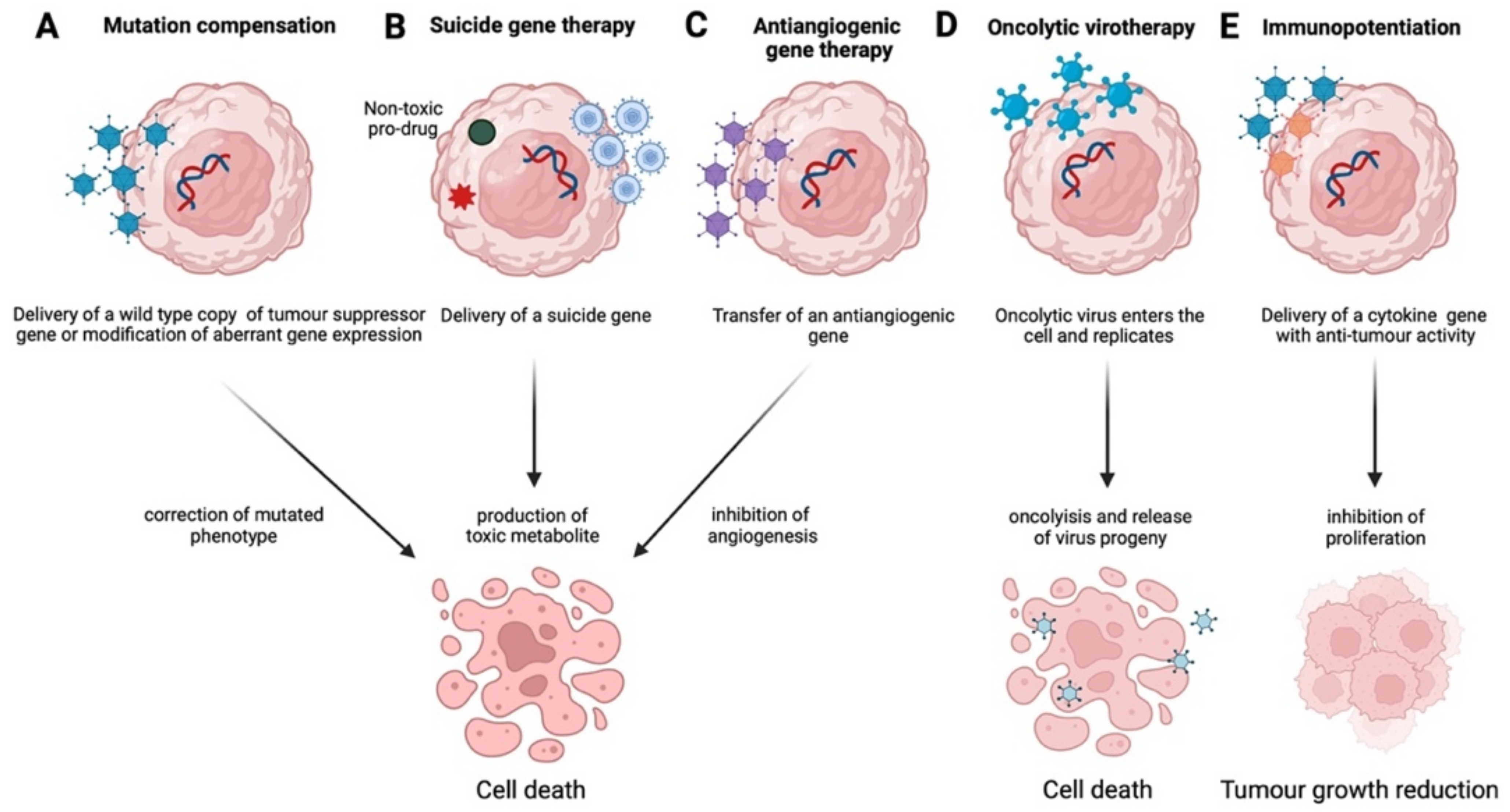
Gene therapy aims to introduce or modify genetic material into target cells, thus altering their function, usually by either restoring a lost function or initiating a new one. Although it was initially employed for the treatment of inherited genetic diseases, gene therapy was soon identified as an effective approach for the treatment of both gynaecological malignancies such as ovarian [2], cervical [3], and endometrial cancer [4] and certain benign gynaecological abnormalities, such as leiomyomas, endometriosis, placental, and embryo implantation disorders [5]. There are two main strategies for specific and efficient gene delivery to cancer and non-cancer cells, and these involve either viral or non-viral systems.
- gene therapy
- gynaecological cancer
- ovarian cancer
- cervical cancer
- viral vectors
- non-malignant gynaecological disorders
1. Gene Delivery Systems
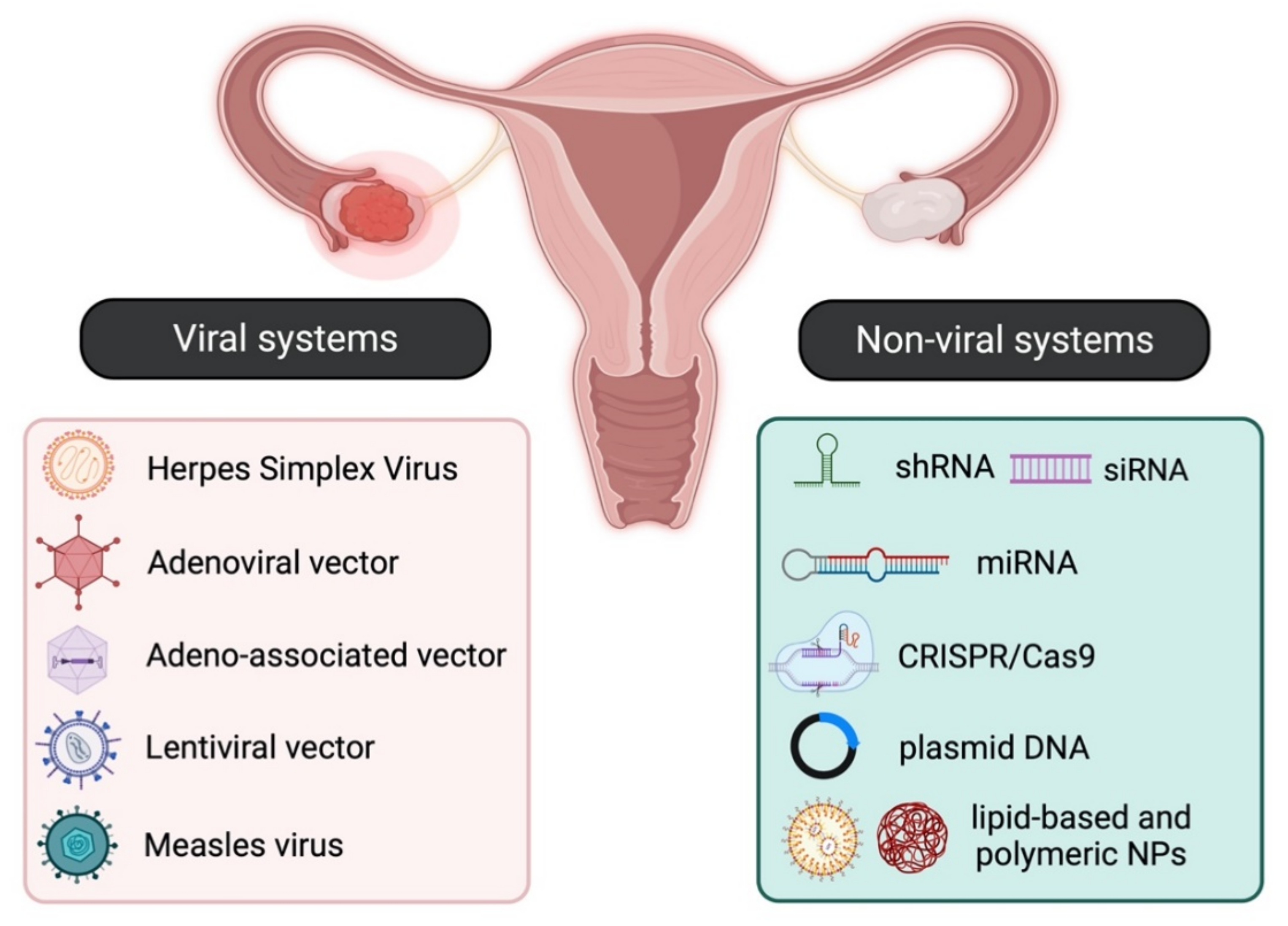
The main strategies for targeted gene therapy approaches for malignant and benign gynaecological disorders employ both viral and non-viral systems (Figure 1).
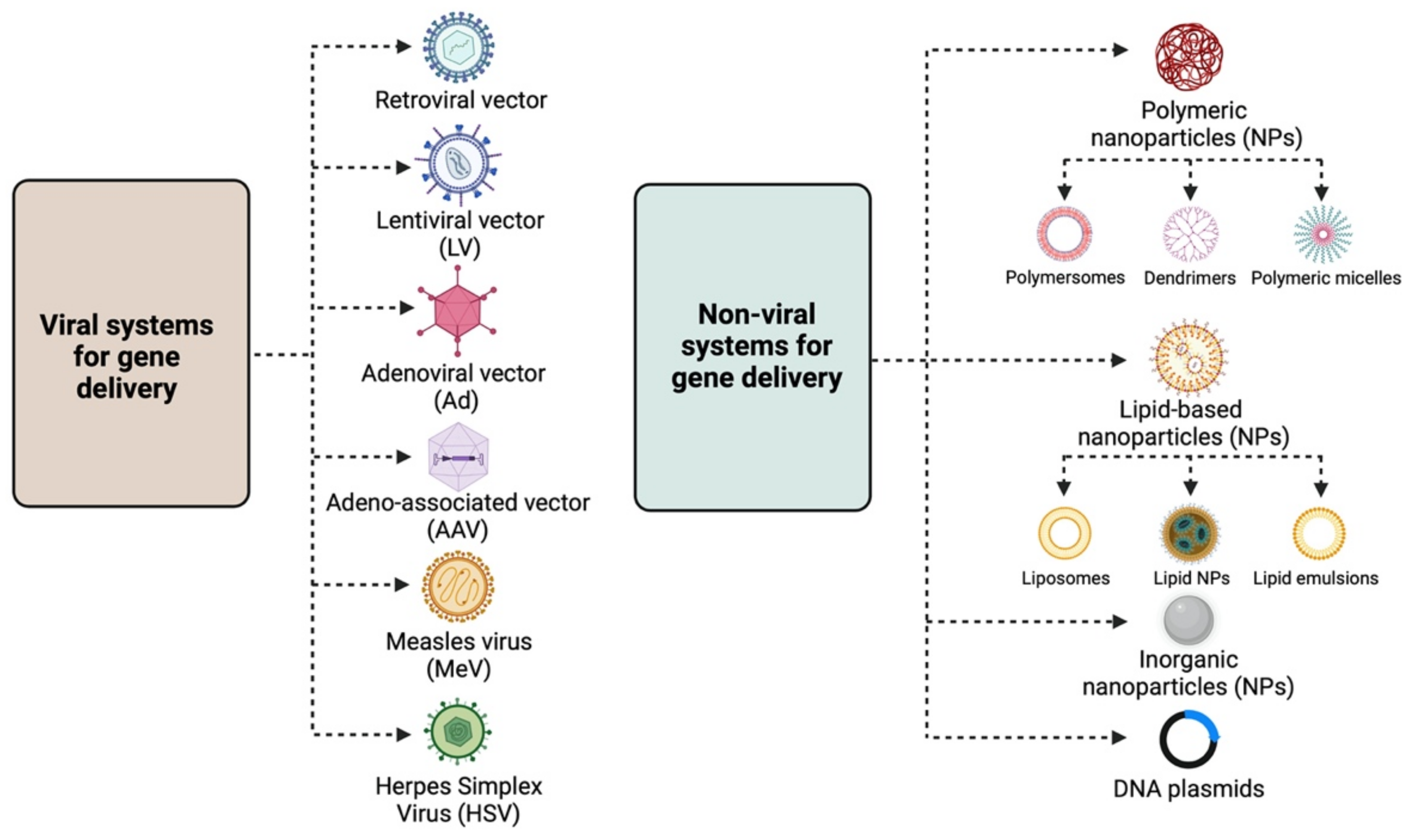
2.1. Viral Systems
1.1. Viral Systems
-
Retroviral vectors: Lentiviral vectors (LVs) are the most widely used retroviruses for therapeutic gene delivery, as they can efficiently transduce both quiescent and dividing cells and thus facilitate safe, efficient, and stable transgene expression [7,8][3][4]. They are enveloped, single-stranded RNA viruses with a packaging capacity of ~9 kb [7][3] and have already been employed in numerous successful clinical trials for the treatment of various diseases, such as hemoglobinopathies, metabolic and immune disorders, and various cancer types, including gynaecological malignancies
21.2.2. Chemical Vectors
-
Polymeric NPs: These are stable, biodegradable, and water-soluble NPs, which make ideal candidates for drug delivery. They exist as monomers or polymers of different structures, with the most common being nanocapsules and nanospheres. According to shape, they are further divided into polymersomes, dendrimers, and micelles. Polymersomes are artificial vesicles with amphiphilic membranes that display increased cargo capacity and effective cytosol delivery [21][17], whereas micelles are quite effective for aqueous drug administration due to their hydrophilic core and hydrophobic coating. Polymeric micelles have already been employed in clinical trials, specifically in a study testing the effective delivery of paclitaxel (PTX), a drug widely used for ovarian cancer treatment [22][18]. Dendrimers are more complex, three-dimensional nanocarriers, commonly used for the delivery of nucleic acids and drugs in cancer therapy, usually in combination with poly amidoamine (PAMAM) and polyethylenimine (PEI) polymers [23][19]. Despite the toxicity associated with the surface amino groups, PAMAM was the first dendrimer used for gene delivery [17][13];
-
Adenoviral vectors (Ads): Adenoviral vectors have been extensively used as viral vector platforms primarily due to their broad tropism and the high transduction efficiency of both quiescent and dividing cells, as well as for their capacity to persist as episomal elements within the target cells [9][5]. They mainly derive from human serotypes-2 (Ad2) and -5 (Ad5) adenoviruses, which are non-enveloped, double-stranded DNA viruses with an icosahedral capsid able to accommodate up to 45 kb linear, double-stranded DNA [10][6]. However, since they are usually associated with potent immune responses [11][7] due to pre-existing immunity, extensive research has focused on generating less immunogenic Ad vectors, employing serotypes with low seroprevalence, such as Ad26 and Ad35 [12][8]. Furthermore, the generation of conditionally replicative adenoviral vectors (CRAds) by introducing tumour-specific promoters into the Ad genome can lead to higher gene expression [13][9]. Regarding gene therapy for malignant and benign gynaecological disorders, Ads have been extensively used as therapeutic vectors in both pre-clinical and clinical studies [9][5] for the delivery of vaccines, tumour suppressor genes, suicide genes, and immunomodulatory genes.
-
Adeno-associated vectors (AAVs): Adeno-associated vectors are single-stranded DNA viruses with an icosahedral capsid of ~5 kb packaging capacity that depend on adenoviruses to complete their life cycle. The current AAV1 and AAV2 serotypes used are characterised by broad tropism, stable episomal persistence, and reduced immunogenicity, and therefore, they have been widely employed in gene therapy approaches [9][5]. Although AAV vectors have not been used yet in clinical gene therapy trials for gynaecological disorders, encouraging data from their pre-clinical assessment presented in this review point toward this direction;
-
Measles virus (MeV): The vaccine strain of MeV is a negative-strand RNA virus with a 16 kb-long genome that presents an attractive oncolytic platform, mainly due to its ability to selectively infect malignant cells, which overexpress the CD46 receptor [14][10]. Due to the fact that the monotherapy oncolytic approach using MeV is usually not sufficient to treat advanced-stage malignancies, combination approaches using radiotherapy or chemotherapy, MeV harbouring with therapeutic or immunomodulatory genes, and the use of carrier cells for MeV delivery into tumour cells can lead to successful therapeutic outcomes for a number of malignancies, including gynaecological cancers [15][11];
-
Herpes Simplex Virus (HSV): HSV-1 and HSV-2 members are enveloped, double-stranded DNA viruses with an icosahedral capsid, which demonstrate attractive vector features, such as a large accommodation capacity, easy production, and high titers [16][12]. Apart from their ability to mediate oncolysis upon delivery of the suicide thymidine kinase (TK) suicide gene into malignant cells, HSV vectors have also been used to deliver immunomodulatory cytokines into tumour cells and thus elicit a strong anti-tumour immune response. Extensive research has focused on the development of safer HSV vectors, carrying transcriptionally active promoter elements that overcome the latency of the viral genome, aiming to expand HSV pre-clinical and clinical applications [16][12].
2.2. Non-Viral Systems
1.2. Non-Viral Systems
The need for non-viral gene delivery systems arose primarily due to the immunogenicity and cytotoxicity concerns raised by some viral vectors, such as Adenoviruses [11][7]. Despite the relatively low transfection efficiency, their use in the field of gene therapy has gained a lot of ground [13,17][9][13]. The main non-viral approaches involve the delivery of the desired gene material, e.g., naked or plasmid DNA, either by physical methods, such as electroporation, ballistic DNA, injection, photoporation, magnetofection, sonoporation, and hydroporation [13[9][14],18], or chemically, by means of polymeric, lipid-based and inorganic nanoparticles (NPs) [13,19][9][15].
21.2.1. Physical Methods
-
Electroporation: The electric pulse creates pores in the cell membrane, allowing the genetic material to enter the target cell. Depending on the target tissue, the electric pulse differs both in strength (high or low) and duration (short or long pulses), with cancer cells usually requiring low field strength (<700 V/cm) with long pulses (milliseconds) [13][9];
-
Injection: The genetic material is directly injected into tissue by means of a needle. It represents the preferred approach for solid tumours; however, it has a relatively low efficiency
-
Lipid-based NPs: They are usually spherical vehicles with an internal aqueous compartment and an external lipid bilayer. They are divided into liposomes or lipoplexes, lipid emulsions (or nanogels), and lipid NPs [17][13]. Due to their positive charge, cationic liposomes bind to the anionic phosphate group of nucleic acids, forming lipoplexes and interacting with cell membranes, and thus, efficiently deliver nucleic acids into the cell [17][13]. Lipid emulsions, which are composed of oil, water, and a surface-active agent, show increased stability and serum resistance, whereas solid lipid NPs can protect nucleic acids from nucleases and are primarily employed for siRNA delivery [20][16];
- .
2. Gene Therapy for Gynaecological Cancers
2.1. Ovarian Cancer
32.1.1. Viral Vectors
Retroviruses
Adenoviruses (Ads) and Adeno-Associated Viruses (AAVs)
These viral systems have been widely used in ovarian cancer gene therapy employed in suicide gene therapy, antiangiogenic, and oncolytic virotherapy, alone or together with immunopotentiation, as well as mutation compensation approaches. With regards to suicide gene therapy, Rawlinson et al. [27][25] developed an adenoviral vector carrying the HSV-TK gene under the tumour-specific promoter HE4 and demonstrated increased cell death by prodrug ganciclovir (GCV) treatment in vitro, in ovarian cancer cell lines. When Zhou et al. applied the above system in vivo in a murine ovarian cancer model, they observed an enhanced anti-tumour effect and increased apoptosis, as well as a reduction in micro-vessel density [28][26]. In a similar approach, but using the NTR-CB1954 system instead, White et al. performed an adenoviral delivery of both NTR and CB1954 in ovarian cancer cells and achieved increased survival in both in vitro and in vivo [29][27].Herpes Simplex Virus (HSV), Measles Virus (MeV), and Vesicular Stomatitis Virus (VSV)
They comprise the main viral systems utilised in oncolytic virotherapy/suicide gene therapy approaches for ovarian cancer gene therapy. More specifically, Hartkopf et al. [45][28] designed a recombinant strain of MeV that carried a fusion cytosine deaminase (CD) protein, which enhanced the chemosensitivity of ovarian cancer cells in 5-fluoroucacil (5-FU) and used it in vitro and in vivo investigations. The autscholars demonstrated the effective infection and lysis of both human ovarian cancer cell lines and primary tumours [45][28]. In another study, Hanauer et al. [39][29], using a bispecific oncolytic MeV, with both HER2 and EpCAM as target receptors, demonstrated a significantly enhanced lysis of tumour cells in xenograft mice, highlighting the superiority of bispecific against monospecific oncolytic viruses [39][29]. Regarding HSV viruses, Goshima et al. [41][30] and Thomas et al. [40][31], in two independent studies, employed HSV in combination with immunostimulatory cytokines, such as GM-CSF and IL-12, respectively. The intraperitoneal injection (IP) of HSV-HF10 in a murine ovarian cancer murine model, together with the anti-tumour effect of GM-CSF, led to a reduction in tumour size and an increase in anti-tumour immune response [41][30].32.1.2. Non-Viral Systems
Plasmids
The use of these approaches has yielded therapeutic effects through suicide gene therapy, immunopotentiation, and mutation compensation strategies. More specifically, Sher et al. [50][32] constructed the hEndoyCD fusion protein, composed of the antiangiogenic endostatin and the Escherichia coli cytosine deaminase (CD) domain which converts 5-fluorocytosine (5-FC) to 5-FU, and delivered it to ovarian cancer cells via a plasmid vector (SV-hEndoyCD). The autscholars reported tumour-specific growth inhibition and increased survival in xenograft mouse models [50][32]. Regarding immunopotentiation approaches, two reports provide evidence of survival improvement [57,58][33][34]. Specifically, Hu et al. employed human umbilical cord CD34+ stem cells transfected with the pIRES2-IL-21-EGFP plasmid, carrying the anti-tumour cytokine IL-21, and demonstrated a therapeutic effect in ovarian cancer xenografts, possibly due to tumour-specific NK cytotoxicity, as a result of elevated levels of IFN-γ and TNF-α. Despite the gradual decrease in IL-21 in mice tumour tissues, overall extended survival was observed in these mice [57][33]. Similarly, Fewell et al. employed a pmIL-12/PPC vehicle carrying the anti-cancer IL-12 cytokine and demonstrated an efficient treatment for disseminated ovarian cancer as a result of the significant VEGF decrease and IL-12 and IFN-γ increase following IP administration [58][34].Nanoparticles (NPs)
The use of these systems, primarily in suicide gene therapy and mutation compensation targeted approaches against ovarian cancer, has also yielded significant results. Specifically, Bai et al. transferred gelonin toxin in ovarian cancer cells using cationic heparin PEI (HPEI) nanogels and managed to reduce cancer cell growth and induce apoptosis [51][35]. Furthermore, Huang et al. [52][36] delivered diphtheria toxin subunit-A (DT-A) DNA in mice with ovarian tumours by means of cationic polymer administered IP, placing it under the control of a human epididymis protein 4 (HE4) promoter, whose activity is increased in ovarian cancer cells. The latter promoter was also used to drive the TK gene, leading to the inhibition of tumour growth and increased survival in mice upon delivery [52][36].miRNAs
Large-scale microarray analysis has highlighted the role of many microRNAs [miR(s)] in different types of cancer, including ovarian and cervical cancer [82][37]. miRNAs in ovarian cancer have shown to have either a tumour-promoting or tumour-suppressing role, depending on whether their expression is up-regulated or down-regulated, respectively, and hence can be employed both as therapeutic targets, as well as biomarkers for diagnosis and/or prognosis [83][38]. Regarding ovarian cancer, Iorio et al. [84][39] performed an initial and comprehensive miRNAs comparison between normal and ovarian cancer tissues and showed that miR-14, mir-199a, miR-200a, miR-200b, and miR-200c were up-regulated in ovarian cancer and thus acted as oncogenic miRs (oncomiRs), while miR-15, miR-16, mir-140, mir-145, mir-199a, and miR-125b1 were down-regulated, pointing toward a tumour-suppressing role (tumour suppressor miRs). Further studies highlighted novel players, such as miR-214, miR-150, miR-140-5P, miR-21, miR-29a, and let-7a and provided more insights into the role of different miRNAs in ovarian cancer.3.2. Cervical Cancer
2.2. Cervical Cancer
Cervical cancer remains the fourth most common and most lethal cancer type in women, despite regular screening and prevention strategies [95][40]. Targeted gene therapy presents a promising approach for the treatment of the specific malignancy and focuses primarily on mutation compensation strategies, suicide gene therapy, oncolytic virotherapy, antiangiogenic strategies, immunopotentiation, and drug resistance therapies [3][41], employing both viral and non-viral systems.32.2.1. Viral Vectors
Lentiviruses
Cervical cancer gene therapy approaches employ LVs primarily for mutation compensation, antiangiogenic, and suicide gene therapy strategies. The restoration of important tumour suppressor genes’ expression, such as tyrosine phosphatase receptor J (PTPRJ), asparaginase and isoaspartyl peptidase 1 (ASRGL1), and homeobox-containing 1 (HMBOX1), has been successfully achieved with LVs. Specifically, Yan et al. [96][42] used pSicoR-PTPRJ LV to overexpress PTPRJ, a tumour suppressor gene whose expression is down-regulated in human cervical cancer tissues and demonstrated significant suppression of cell viability, migration, and growth in HPV-negative C33A cells. On the contrary, the knock-down of PTPRJ expression in the above cervical cancer cell line led to increased resistance to 5-FU-mediated apoptosis, verifying the importance of elevated PTPRJ expression for cervical cancer prevention. Moreover, Zhou et al. used a lentiviral shRNA to knock-down HMBOX1 expression in HeLa and C33A cancer cells and demonstrated increased radiosensitivity as a result of telomere shortening [97][43]. Lastly, the knock-down of ASRGL1 expression in SiHa cells by means of shRNA led to decreased proliferation, possibly through the reduced expression of CDK2 and cyclin A2 and the induction of apoptosis [98][44], which was characterised by the increased expression of the pro-apoptotic Bax and the decreased expression of anti-apoptotic Bcl-2. Regarding the antiangiogenic approach, Qi et al. used a lentiviral shRNA-VEGF construct to knock down VEGF expression in vitro and in vivo in nude mice and was able to inhibit tumour growth and tumour radiosensitivity [99][45]. One of the most promising approaches for cervical cancer gene therapy using lentiviruses is the genetic modification of T-cell receptors (TCRs) to target tumour-specific antigens. Based on the above, Jin et al. developed E7-specific T cells and achieved regression of HPV-positive mouse tumours [100][46]. The specific approach is currently under clinical trial NCT02379520 and is being used on patients with metastatic cervical cancer [101][47].Adenoviruses (Ad) and Adeno-Associated Viruses (AAV)
They comprise the majority of viral vectors used for cervical cancer gene therapy, often utilised for tumour suppressor gene restoration or blocking of oncogenic expression. The p53 gene, a key regulator of cell proliferation, apoptosis, and genetic stability [141][48], plays a fundamental role in most gynaecological cancers and has therefore been a major candidate for most targeted gene therapy approaches [78][49]. More specifically, since the p53 gene is often inactivated by HPV E6 protein in most cervical cancers [142][50]; either its delivery or inhibition of the E6 protein could result in significant antitumour effects. Indeed, Su et al. employing Genidicine®, a gene therapy product approved in 2003 by the China Food and Drug Administration (CFDA) for head and neck cancer gene therapy [143][51], showed that the injection of a recombinant human adenovirus engineered to express wild-type p53 gene (rArd-p53) in cervical cancer patients, may lead to an increased 5-year overall survival rate [105][52]. Moreover, Kajitani et al., using siRNA for E6 protein in HeLa cells, demonstrated successful p53 transduction following adenovirus treatment [106][53]. When the above transduction in HeLa cells was combined with PTX, it led to enhanced growth inhibition and apoptosis, as demonstrated by Liu et al. [38][54].32.2.2. Non-Viral Systems
Nanoparticles (NPs)
Gene therapy strategies by means of nanoparticles focus primarily on mutation compensation and immunopotentiation strategies. Regarding the former approach, Liu et al. [144][55] developed polyethylene glycol-polylactic acid (PEG-PLA) NPs linked to folate and targeted cancer cells through the folate receptor α (FRα), a membrane-bound protein mediating folate uptake. Although the autscholars observed enhanced gene transfection efficiency, higher compared to naked DNA, and reduced cytotoxicity, the strategies targeting FRα receptors are hampered by its heterogeneous expression among cervical cancer patients [145][56]. A few years later, Yang et al. overexpressed FRα, previously shown to be highly expressed in cervical cancer, using an FRα-targeted liposome (FLP) to deliver a pigment epithelium-derived factor (PEDF) gene into HeLa cells and observed significant anti-tumour activity, as demonstrated by significant growth inhibition, the suppression of adhesion and invasion, and cancer cell migration in vitro [130][57].Plasmids
The use of different plasmids in cervical cancer gene therapy approaches focuses on mutational compensation, antiangiogenesis, immunopotentiation and chemoresistance. With regards to blocking oncogenic expression, Hu et al. employed the genome editing approach using both the CRISPR/Cas9 and transcription-activator-like nucleases (TALEN) systems to disrupt the HPV16 E7 oncoprotein coding gene [122][58]. The data documented induction of apoptosis and growth inhibition in HPV16-positive human cancer cells. The latter approach is being studied in Phase I clinical trials for the treatment of CIN-1 patients with HPV16 and HPV18 infection [101][47]. Recently, two groups demonstrated the therapeutic effect of targeting HPV E6 and E7 genes. Ling et al., using the CRISPR/Cas9 approach to delete the HPV18 E6 and E7 genes, achieved robust knock-out of these proteins and increased apoptosis and tumour size reduction [119][59]. In an attempt to compare the efficiency of CRISPR/Cas9 with the established TALEN approach, Gao et al. employed the former system against the HPV16 E7 gene and succeeded in reverting cervical carcinogenesis, both in vitro and in vivo [124][60]. Another tumour suppressor gene with clinical importance in cervical cancer gene therapy is the retinoblastoma protein zinc finger gene 1 (RIZ1) since it can induce apoptosis and cell cycle arrest. Cheng et al. demonstrated that overexpression of RIZ1 expression in HPV16-positive cervical cancer cells could lead to impaired cell proliferation and increased apoptosis [131][61].miRNAs
miRNAs involved in cervical cancer induction and development are classified into oncogenic miRNAs (oncomiRs) and tumour suppressor miRNAs (tumour suppressor miRs) [147[62][63],148], and therefore, their down-regulation or overexpression, respectively, can be curative, while their presence in patients’ serum is considered as an important tool for cancer diagnosis and prognosis. More specifically and regarding oncomiRs, the most important ones include miR-10a, miR-19, miR-20, miR-21, miR-133b, and miR-886-5p [147][62]. Long et al. showed that miR-10a suppresses cell adhesion molecule L1, such as (CHL1), and thus leads to enhancement of tumour growth, metastasis, and invasion [149][64], while miR-19 is overexpressed in cervical cancer, and its silencing in SiHa cells led to a reduction in the proliferation and induction of apoptosis, through the up-regulation of Bax and down-regulation of Bcl-2 expression [150][65]. MiR-20 is a positive regulator of tyrosine kinase non-receptor 2 (TNKS2), an oncogene involved in metastasis and invasion [151][66]. In contrast, miR-21, which is the most well-known oncogenic miRNA, acts as a negative regulator of the tumour suppression gene programmed cell death 4 (PDCD4), which normally inhibits cell proliferation and induces apoptosis [152][67], thus promoting tumour growth. Silencing by means of siRNA in cervical cancer cell lines led to inhibition of cell proliferation and induction of cell death by autophagy and caspase 3/7-mediated apoptosis [153][68]. Moreover, through targeting and regulating CCL20, a gene involved in tumour differentiation and metastasis, miR-20 was implicated in cervical squamous carcinogenesis [152][67].3.3. Endometrial Cancer
2.3. Endometrial Cancer
Despite being usually curable following surgery, endometrial cancer still presents as one of the most common female reproductive tract cancer types [4][69]. Occasionally aggressive tumours, such as uterine papillary serous carcinomas (UPSC), are observed, with most of them demonstrating aberrant expression of p53 [164][70]. Gene therapy strategies toward endometrial cancer involve both viral and non-viral systems, with the former employing primarily adenoviral and retroviral vectors and the latter plasmid DNA/RNA. analysis has highlighted the role of many microRNAs [miR(s)] in different types of cancer, including ovarian and cervical cancer [82][37]. miRNAs in ovarian cancer have shown to have either a tumour-promoting or tumour-suppressing role, depending on whether their expression is up-regulated or down-regulated, respectively, and hence can be employed both as therapeutic targets, as well as biomarkers for diagnosis and/or prognosis [83][38]. Regarding ovarian cancer, Iorio et al. [84][39] performed an initial and comprehensive miR32.3.1. Viral Vectors
Ramondetta et al. performed an adenovirus-mediated expression of p53 or p21 in a papillary serous endometrial carcinoma cell line and demonstrated growth inhibition and apoptotic cell death [165][71]. Similarly, Ural et al. performed an in vitro suicide gene therapy approach using HSV-TK and documented inhibition of endometrial cancer cell growth [166][72]. A similar approach, but with the use of the pNF-κB plasmid, along with the gonadotropin-releasing hormone receptor (GnRH-R) agonist triptorelin and the prodrug GCV, resulted in reduced cancer cell growth, both in vitro and in vivo in mice [167][73]. Recently, Xia et al. [168][74] employed next-generation sequencing (NGS) in four out of the twelve patients enrolled in the clinical trial using the Ad-p53 vector, Genidicine® [105][52] and observed reduced p53 expression in the tumours of three patients, all carrying mutations in tumour protein p53 CREB binding protein (CREBBP), cyclin-dependent kinase inhibitor 2A (CDKN2A), LYN proto-oncogene (LYN) and Janus kinase 2 (JAK2) genes. These data provide strong evidence that NGS can aid in the recruitment of suitable patients for Ad-53 uterine gene therapy.2.3.2. Non-Viral Systems
Effective gene transfer with a significant inhibition of cancer cells in vitro was also achieved with non-viral approaches. Specifically, Maurice-Duelli et al. demonstrated that the transfer of PTEN, a frequently mutated gene in endometrial cancer [169][75], using PEI-photochemical irradiation could lead to a 44% inhibition in cancer cell growth [170][76]. Moreover, the role of certain miRNAs in endometrial cancer has gained a lot of ground, highlighting their potential diagnostic and therapeutic value. In a detailed review, Banno et al. [171][77] highlighted the role of several miRNAs differentially expressed in endometrial cancer. Specifically, miR-185, miR-106a, miR-181a, miR-210, miR-423, miR-107, miR-let7c, miR-205, miR-449, and miR-429 were found up-regulated, while miR-let7e, miR-221, miR-30c, miR-152, miR-193, miR-204, miR-99b and miR-193b were significantly down-regulated, suggesting a tumorigenic or tumour-suppressing activity, respectively [171][77]. miR-129-2 and miR-152 are involved in the development of endometrial cancer via DNA methylation; miR-125b, mir-30c, miR-200b/c, and miR-429 are related to cisplatin resistance, while miR-125b, miR-30c, miR-194 and miR-34b regulate proliferation, metastasis and invasion of endometrial cancer cells [171][77]. Similarly, in a recent systematic review, Donkers et al. [172][78] highlighted the importance of miR-205, miR-200 family (miR-200a, miR-200b, and miR-200c), miR-135b, miR-182, miR-183, and miR-223 in endometrial cancer prognosis [172][78]. As the above miRNAs are found to be up-regulated in most endometrial cancers, the down-regulation of their expression may have therapeutic outcomes. Regarding miRNAs, such as miR-137, miR-129-3p, and miR-410, whose expression is down-regulated in endometrial cancer, the autscholars observed little to no consensus [172][78], and therefore, their role in both predicting and treating endometrial cancer remains vague.3. Gene Therapy for Benign Gynaecological Disorders
3.1. Uterine Leiomyomas or Fibroids
They are the most common benign tumours in reproductive-age women [173][79] and represent an attractive target for gene therapy, primarily due to their localised nature and slow growth rate [5,174][1][80]. The first attempt to employ gene therapy for uterine leiomyoma treatment came from Niu et al. 1998, who used a non-viral approach to deliver the suicide TK gene into leiomyoma cells [175][81]. Although the autscholars demonstrated significant cell death despite the low percentage of transfected leiomyoma cells, the above approach could only be applied in vitro. On the contrary, the use of improved Ads [174][80] with a leiomyoma-specific expression of critical therapeutic genes, such as DNER [176][82] and HSV1-TK [5][1], appear quite promising and present good candidates for human clinical trials. Furthermore, targeted and transduction-efficient Ads, such as Adenovirus-human somatostatin receptor subtype 2-arginine, glycine, and aspartate-thymidine kinase (Ad-SSTR-RGD-TK), given in combination with GCV, show promising results, both in vitro [177][83] and in vivo [178][84], as they lead to a significant reduction in proliferation and leiomyomas size, the induction of apoptosis, and the inhibition of angiogenic- and extracellular matrix-related genes. Recently, C-terminal Src kinase (CSK2) [179][85] and high mobility group AT-hook 2 (HMGA2) [180][86] were highlighted as key players in leiomyomas tumorigenesis. Specifically, CSK2 was shown to be highly expressed in uterine leiomyosarcomas compared to leiomyomas, and its overexpression was associated with tumour progression and poor prognosis, while CSK2 silencing using siRNA led to the inhibition of proliferation and cell cycle progression, decreased migration, invasion, and colony formation [179][85]. Additionally, the overexpression of HMGA2 in leiomyomas was shown to be associated with increased vasculature density, demonstrated by the increased expression of angiogenic factors and receptors, such as VEGFA, EGF, bFGF, TGFα, VEGFR1, and VEGFR2, which likely contribute to tumour growth [180][86]. Its important role in angiogenesis appears to be through IGF2BP2-mediated pAKT activity [180][86].3.2. Endometriosis
It is a chronic, estrogen-dependent disease characterised by the presence of endometrial tissue outside the uterine cavity, often associated with subfertility [5,198][1][87]. In the early 2000s, the above benign gynaecological abnormality made its way to gene therapy applications (Table 3 and Figure 5). Specifically, Dabrosin et al. using a murine model of endometriosis and employing an adenovirus expressing the murine angiostatin gene managed to completely eradicate endometriotic lesions within two weeks [181][88]. A similar antiangiogenic strategy was followed by Sun et al., who used an AAV carrying endostatin (rAAv-endostatin-EGFP) and demonstrated the inhibition of angiogenesis in endometriotic lesions both in vitro and in vivo [182][89]. Similarly, but using an endostatin plasmid/PAMAM dendrimer instead, Wang et al. also demonstrated inhibition of endometriosis development following transfection [185][90]. Following the above, Othman and co-workers studied the effects of DNER gene transfer into endometriosis cells using an Ad-DNER vector and showed that it could induce significant cell death and reduce pro-inflammatory and angiogenic cytokine production by the specific cells [183][91]. Both of the above studies used an Ad5 viral vector; however, CRADs, such as Ad-SLP1-luc, Ad-hepanarase-luc [184][92], and Ad-sft-1 [186][93], achieved higher reporter gene expression and, therefore, may constitute better candidates for the gene therapy of endometriosis.3.3. Placental Disorders
The potential contribution of gene therapy for placental abnormalities and functional restoration deficiencies was first introduced by Senut et al. [201][94] by injecting genetically modified cells into rat placenta, which demonstrated the increased secretion of gene products in fetal circulation. Furthermore, Xing et al. managed to successfully transfect rat placenta by either systemic administration or intra-placental needle injection of adenoviruses without altering the fetal genome [191][95]. However, as intra-placental needle injection may result in fetal-maternal barrier breakdown, Heikilla and co-workers performed a catheter-mediated intravascular gene transfer with adenoviruses, plasmid/liposomes and plasmid/PEI complexes and demonstrated that placental trophoblastic cells could be efficiently transfected with adenoviruses when delivered directly into uterine arteries [192][96]. On the contrary, and despite their ability to transfect fetal membranes and the basal plate more efficiently than adenoviruses, plasmid/PEI and plasmid/liposome complexes were not as efficient in transfecting placental trophoblastic cells [192][96].3.4. Embryo Implantation Disorders
The proof of principle that genetic manipulation of the adult uterine endometrium has therapeutic potential against implantation disorders came from Bagot et al. in early 2000 [196][97]. The autscholars first reported that the alteration of maternal Hoxa10 expression, previously shown to be expressed in human endometrium during implantation [202][98] by in vivo gene transfection, affects implantation in mice [196][97]. Based on this finding, they overexpressed the maternal homeobox A10 (Hoxa10) gene using a pcDNA3.1(+)/HOXA10a, a liposome plasmid that constitutively expresses Hoxa10, and achieved a significant increase in litter size. All of the mice pups were born following a normal gestation of 17–20 days, were normal in size and exhibited no morphological abnormalities.4. Conclusions
It is clear that targeted gene therapy approaches, such as mutation compensation, antiangiogenesis, immunopotentiation, suicide gene therapy, and oncolytic virotherapy, have yielded promising results, employing both viral and non-viral systems. However, most of these strategies either remain in the preclinical phase or show reduced effectiveness as monotherapies and usually require a combination of chemotherapy and radiotherapy to demonstrate a therapeutic outcome. Furthermore, gene therapy in combination with immunotherapy, e.g., targeting CTLA-4 and PD-1, or with the emerging therapeutic angiogenesis inhibitors, may result in increased effectiveness, especially for ovarian cancer in advanced stages, where still no effective therapies exist. Moreover, there is increasing evidence for the therapeutic potential of CAR T-cell technology also, and clinical trials are currently assessing its efficacy, primarily in ovarian cancer, by recruiting patients [101][47]. The continuous development of safe and effective vectors, the discovery of novel diagnostic markers, such as miRNAs discussed above, or of new players, such as long non-coding RNAs (lncRNAs) [211][99] and circular RNAs (circRNAs) [212][100] and in combination with more clinical trials beyond Phase I/II are expected to lead to more effective and radical therapeutic outcomes.
References
- Hassan, M.H.; Othman, E.E.; Hornung, D.; Al-Hendy, A. Gene therapy of benign gynecological diseases. Adv. Drug Deliv. Rev. 2009, 61, 822–835.
- Brooks, R.A.; Mutch, D.G. Gene therapy in gynecological cancer. Expert Rev. Anticancer Ther. 2006, 6, 1013–1032.
- Naldini, L.; Blomer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267.
- Naldini, L.; Blomer, U.; Gage, F.H.; Trono, D.; Verma, I.M. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA 1996, 93, 11382–11388.
- Bulcha, J.T.; Wang, Y.; Ma, H.; Tai, P.W.L.; Gao, G. Viral vector platforms within the gene therapy landscape. Signal Transduct. Target 2021, 6, 53.
- Greber, U.F. Adenoviruses—Infection, pathogenesis and therapy. FEBS Lett. 2020, 594, 1818–1827.
- Muruve, D.A. The innate immune response to adenovirus vectors. Hum. Gene Ther. 2004, 15, 1157–1166.
- Chen, H.; Xiang, Z.Q.; Li, Y.; Kurupati, R.K.; Jia, B.; Bian, A.; Zhou, D.M.; Hutnick, N.; Yuan, S.; Gray, C.; et al. Adenovirus-based vaccines: Comparison of vectors from three species of adenoviridae. J. Virol. 2010, 84, 10522–10532.
- Ramamoorth, M.; Narvekar, A. Non viral vectors in gene therapy—An overview. J. Clin. Diagn. Res. 2015, 9, GE01.
- Lin, L.T.; Richardson, C.D. The Host Cell Receptors for Measles Virus and Their Interaction with the Viral Hemagglutinin (H) Protein. Viruses 2016, 8, 250.
- Engeland, C.E.; Ungerechts, G. Measles Virus as an Oncolytic Immunotherapy. Cancers 2021, 13, 544.
- Lachmann, R. Herpes simplex virus-based vectors. Int. J. Exp. Pathol. 2004, 85, 177–190.
- Zu, H.; Gao, D. Non-viral Vectors in Gene Therapy: Recent Development, Challenges, and Prospects. AAPS J. 2021, 23, 78.
- Sung, Y.K.; Kim, S.W. Recent advances in the development of gene delivery systems. Biomater. Res. 2019, 23, 8.
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug. Discov. 2021, 20, 101–124.
- Al-Dosari, M.S.; Gao, X. Nonviral gene delivery: Principle, limitations, and recent progress. AAPS J. 2009, 11, 671–681.
- Zelmer, C.; Zweifel, L.P.; Kapinos, L.E.; Craciun, I.; Guven, Z.P.; Palivan, C.G.; Lim, R.Y.H. Organelle-specific targeting of polymersomes into the cell nucleus. Proc. Natl. Acad. Sci. USA 2020, 117, 2770–2778.
- Lee, S.W.; Kim, Y.M.; Cho, C.H.; Kim, Y.T.; Kim, S.M.; Hur, S.Y.; Kim, J.H.; Kim, B.G.; Kim, S.C.; Ryu, H.S.; et al. An Open-Label, Randomized, Parallel, Phase II Trial to Evaluate the Efficacy and Safety of a Cremophor-Free Polymeric Micelle Formulation of Paclitaxel as First-Line Treatment for Ovarian Cancer: A Korean Gynecologic Oncology Group Study (KGOG-3021). Cancer Res. Treat. 2018, 50, 195–203.
- Palmerston Mendes, L.; Pan, J.; Torchilin, V.P. Dendrimers as Nanocarriers for Nucleic Acid and Drug Delivery in Cancer Therapy. Molecules 2017, 22, 1401.
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167.
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061.
- Oswald, A.J.; Gourley, C. Low-grade epithelial ovarian cancer: A number of distinct clinical entities? Curr. Opin. Oncol. 2015, 27, 412–419.
- Ayen, A.; Jimenez Martinez, Y.; Marchal, J.A.; Boulaiz, H. Recent Progress in Gene Therapy for Ovarian Cancer. Int. J. Mol. Sci. 2018, 19, 1930.
- Shi, X.X.; Zhang, B.; Zang, J.L.; Wang, G.Y.; Gao, M.H. CD59 silencing via retrovirus-mediated RNA interference enhanced complement-mediated cell damage in ovary cancer. Cell. Mol. Immunol. 2009, 6, 61–66.
- Rawlinson, J.W.; Vaden, K.; Hunsaker, J.; Miller, D.F.; Nephew, K.P. Adenoviral-delivered HE4-HSV-tk sensitizes ovarian cancer cells to ganciclovir. Gene Ther. Mol. Biol. 2013, 15, 120–130.
- Zhou, X.L.; Shi, Y.L.; Li, X. Inhibitory effects of the ultrasound-targeted microbubble destruction-mediated herpes simplex virus-thymidine kinase/ganciclovir system on ovarian cancer in mice. Exp. Ther. Med. 2014, 8, 1159–1163.
- White, C.L.; Menghistu, T.; Twigger, K.R.; Searle, P.F.; Bhide, S.A.; Vile, R.G.; Melcher, A.A.; Pandha, H.S.; Harrington, K.J. Escherichia coli nitroreductase plus CB1954 enhances the effect of radiotherapy in vitro and in vivo. Gene Ther. 2008, 15, 424–433.
- Hartkopf, A.D.; Bossow, S.; Lampe, J.; Zimmermann, M.; Taran, F.A.; Wallwiener, D.; Fehm, T.; Bitzer, M.; Lauer, U.M. Enhanced killing of ovarian carcinoma using oncolytic measles vaccine virus armed with a yeast cytosine deaminase and uracil phosphoribosyltransferase. Gynecol. Oncol. 2013, 130, 362–368.
- Hanauer, J.R.; Gottschlich, L.; Riehl, D.; Rusch, T.; Koch, V.; Friedrich, K.; Hutzler, S.; Prufer, S.; Friedel, T.; Hanschmann, K.M.; et al. Enhanced lysis by bispecific oncolytic measles viruses simultaneously using HER2/neu or EpCAM as target receptors. Mol. Ther. Oncolytics 2016, 3, 16003.
- Goshima, F.; Esaki, S.; Luo, C.; Kamakura, M.; Kimura, H.; Nishiyama, Y. Oncolytic viral therapy with a combination of HF10, a herpes simplex virus type 1 variant and granulocyte-macrophage colony-stimulating factor for murine ovarian cancer. Int. J. Cancer 2014, 134, 2865–2877.
- Thomas, E.D.; Meza-Perez, S.; Bevis, K.S.; Randall, T.D.; Gillespie, G.Y.; Langford, C.; Alvarez, R.D. IL-12 Expressing oncolytic herpes simplex virus promotes anti-tumor activity and immunologic control of metastatic ovarian cancer in mice. J. Ovarian Res. 2016, 9, 70.
- Sher, Y.P.; Chang, C.M.; Juo, C.G.; Chen, C.T.; Hsu, J.L.; Lin, C.Y.; Han, Z.; Shiah, S.G.; Hung, M.C. Targeted endostatin-cytosine deaminase fusion gene therapy plus 5-fluorocytosine suppresses ovarian tumor growth. Oncogene 2013, 32, 1082–1090.
- Hu, W.; Wang, J.; Dou, J.; He, X.; Zhao, F.; Jiang, C.; Yu, F.; Hu, K.; Chu, L.; Li, X.; et al. Augmenting therapy of ovarian cancer efficacy by secreting IL-21 human umbilical cord blood stem cells in nude mice. Cell Transplant. 2011, 20, 669–680.
- Fewell, J.G.; Matar, M.M.; Rice, J.S.; Brunhoeber, E.; Slobodkin, G.; Pence, C.; Worker, M.; Lewis, D.H.; Anwer, K. Treatment of disseminated ovarian cancer using nonviral interleukin-12 gene therapy delivered intraperitoneally. J. Gene Med. 2009, 11, 718–728.
- Bai, Y.; Gou, M.; Yi, T.; Yang, L.; Liu, L.; Lin, X.; Su, D.; Wei, Y.; Zhao, X. Efficient Inhibition of Ovarian Cancer by Gelonin Toxin Gene Delivered by Biodegradable Cationic Heparin-polyethyleneimine Nanogels. Int. J. Med. Sci. 2015, 12, 397–406.
- Huang, Y.H.; Zugates, G.T.; Peng, W.; Holtz, D.; Dunton, C.; Green, J.J.; Hossain, N.; Chernick, M.R.; Padera, R.F., Jr.; Langer, R.; et al. Nanoparticle-delivered suicide gene therapy effectively reduces ovarian tumor burden in mice. Cancer Res. 2009, 69, 6184–6191.
- Lu, J.; Getz, G.; Miska, E.A.; Alvarez-Saavedra, E.; Lamb, J.; Peck, D.; Sweet-Cordero, A.; Ebert, B.L.; Mak, R.H.; Ferrando, A.A.; et al. MicroRNA expression profiles classify human cancers. Nature 2005, 435, 834–838.
- Chen, S.N.; Chang, R.; Lin, L.T.; Chern, C.U.; Tsai, H.W.; Wen, Z.H.; Li, Y.H.; Li, C.J.; Tsui, K.H. MicroRNA in Ovarian Cancer: Biology, Pathogenesis, and Therapeutic Opportunities. Int. J. Environ. Res. Public Health 2019, 16, 1510.
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.G.; Alder, H.; et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007, 67, 8699–8707.
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424.
- Ayen, A.; Jimenez Martinez, Y.; Boulaiz, H. Targeted Gene Delivery Therapies for Cervical Cancer. Cancers 2020, 12, 1301.
- Yan, C.M.; Zhao, Y.L.; Cai, H.Y.; Miao, G.Y.; Ma, W. Blockage of PTPRJ promotes cell growth and resistance to 5-FU through activation of JAK1/STAT3 in the cervical carcinoma cell line C33A. Oncol. Rep. 2015, 33, 1737–1744.
- Zhou, S.; Xiao, Y.; Zhuang, Y.; Liu, Y.; Zhao, H.; Yang, H.; Xie, C.; Zhou, F.; Zhou, Y. Knockdown of homeobox containing 1 increases the radiosensitivity of cervical cancer cells through telomere shortening. Oncol. Rep. 2017, 38, 515–521.
- Lv, X.F.; Hong, H.Q.; Liu, L.; Cui, S.H.; Ren, C.C.; Li, H.Y.; Zhang, X.A.; Zhang, L.D.; Wei, T.X.; Liu, J.J.; et al. RNAimediated downregulation of asparaginaselike protein 1 inhibits growth and promotes apoptosis of human cervical cancer line SiHa. Mol. Med. Rep. 2018, 18, 931–937.
- Qi, L.; Xing, L.N.; Wei, X.; Song, S.G. Effects of VEGF suppression by small hairpin RNA interference combined with radiotherapy on the growth of cervical cancer. Genet. Mol. Res. 2014, 13, 5094–5106.
- Jin, B.Y.; Campbell, T.E.; Draper, L.M.; Stevanovic, S.; Weissbrich, B.; Yu, Z.; Restifo, N.P.; Rosenberg, S.A.; Trimble, C.L.; Hinrichs, C.S. Engineered T cells targeting E7 mediate regression of human papillomavirus cancers in a murine model. JCI Insight 2018, 3, e99488.
- U.S National Library of Medicine. ClinicalTrials.gov. Available online: https://clinicaltrials.gov (accessed on 29 May 2022).
- Li, Y.; Zhang, M.C.; Xu, X.K.; Zhao, Y.; Mahanand, C.; Zhu, T.; Deng, H.; Nevo, E.; Du, J.Z.; Chen, X.Q. Functional Diversity of p53 in Human and Wild Animals. Front. Endocrinol. 2019, 10, 152.
- Nakamura, M.; Obata, T.; Daikoku, T.; Fujiwara, H. The Association and Significance of p53 in Gynecologic Cancers: The Potential of Targeted Therapy. Int. J. Mol. Sci. 2019, 20, 5482.
- Doorbar, J. Molecular biology of human papillomavirus infection and cervical cancer. Clin. Sci. 2006, 110, 525–541.
- Jia, H.; Kling, J. China offers alternative gateway for experimental drugs. Nat. Biotechnol. 2006, 24, 117–118.
- Su, X.; Chen, W.J.; Xiao, S.W.; Li, X.F.; Xu, G.; Pan, J.J.; Zhang, S.W. Effect and Safety of Recombinant Adenovirus-p53 Transfer Combined with Radiotherapy on Long-Term Survival of Locally Advanced Cervical Cancer. Hum. Gene Ther. 2016, 27, 1008–1014.
- Kajitani, K.; Honda, K.; Terada, H.; Yasui, T.; Sumi, T.; Koyama, M.; Ishiko, O. Human Papillomavirus E6 Knockdown Restores Adenovirus Mediated-estrogen Response Element Linked p53 Gene Transfer in HeLa Cells. Asian Pac. J. Cancer Prev. 2015, 16, 8239–8245.
- Liu, Y.G.; Zheng, X.L.; Liu, F.M. The mechanism and inhibitory effect of recombinant human P53 adenovirus injection combined with paclitaxel on human cervical cancer cell HeLa. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1037–1042.
- Liu, B.; Han, S.M.; Tang, X.Y.; Han, L.; Li, C.Z. Cervical cancer gene therapy by gene loaded PEG-PLA nanomedicine. Asian. Pac. J. Cancer Prev. 2014, 15, 4915–4918.
- Wu, M.; Gunning, W.; Ratnam, M. Expression of folate receptor type alpha in relation to cell type, malignancy, and differentiation in ovary, uterus, and cervix. Cancer Epidemiol. Biomarkers Prev. 1999, 8, 775–782.
- Yang, Y.; He, L.; Liu, Y.; Xia, S.; Fang, A.; Xie, Y.; Gan, L.; He, Z.; Tan, X.; Jiang, C.; et al. Promising Nanocarriers for PEDF Gene Targeting Delivery to Cervical Cancer Cells Mediated by the Over-expressing FRalpha. Sci. Rep. 2016, 6, 32427.
- Hu, Z.; Ding, W.; Zhu, D.; Yu, L.; Jiang, X.; Wang, X.; Zhang, C.; Wang, L.; Ji, T.; Liu, D.; et al. TALEN-mediated targeting of HPV oncogenes ameliorates HPV-related cervical malignancy. J. Clin. Investig. 2015, 125, 425–436.
- Ling, K.; Yang, L.; Yang, N.; Chen, M.; Wang, Y.; Liang, S.; Li, Y.; Jiang, L.; Yan, P.; Liang, Z. Gene Targeting of HPV18 E6 and E7 Synchronously by Nonviral Transfection of CRISPR/Cas9 System in Cervical Cancer. Hum. Gene Ther. 2020, 31, 297–308.
- Gao, C.; Wu, P.; Yu, L.; Liu, L.; Liu, H.; Tan, X.; Wang, L.; Huang, X.; Wang, H. The application of CRISPR/Cas9 system in cervical carcinogenesis. Cancer Gene Ther. 2021, 29, 466–474.
- Cheng, H.Y.; Zhang, T.; Qu, Y.; Shi, W.J.; Lou, G.; Liu, Y.X.; Zhang, Y.Y.; Cheng, L. Synergism between RIZ1 gene therapy and paclitaxel in SiHa cervical cancer cells. Cancer Gene Ther. 2016, 23, 392–395.
- Banno, K.; Iida, M.; Yanokura, M.; Kisu, I.; Iwata, T.; Tominaga, E.; Tanaka, K.; Aoki, D. MicroRNA in cervical cancer: OncomiRs and tumor suppressor miRs in diagnosis and treatment. Sci. World J. 2014, 2014, 178075.
- Tornesello, M.L.; Faraonio, R.; Buonaguro, L.; Annunziata, C.; Starita, N.; Cerasuolo, A.; Pezzuto, F.; Tornesello, A.L.; Buonaguro, F.M. The Role of microRNAs, Long Non-coding RNAs, and Circular RNAs in Cervical Cancer. Front Oncol. 2020, 10, 150.
- Long, M.J.; Wu, F.X.; Li, P.; Liu, M.; Li, X.; Tang, H. MicroRNA-10a targets CHL1 and promotes cell growth, migration and invasion in human cervical cancer cells. Cancer Lett. 2012, 324, 186–196.
- Wang, Y.; Wang, Y.; Zhong, W.; Gulina, K. Correlation between miR-19a inhibition and radiosensitivity in SiHa cervical cancer cells. J. BUON 2017, 22, 1505–1508.
- Kang, H.W.; Wang, F.; Wei, Q.; Zhao, Y.F.; Liu, M.; Li, X.; Tang, H. miR-20a promotes migration and invasion by regulating TNKS2 in human cervical cancer cells. FEBS Lett. 2012, 586, 897–904.
- Yao, Q.; Xu, H.; Zhang, Q.Q.; Zhou, H.; Qu, L.H. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem. Biophys. Res. Commun. 2009, 388, 539–542.
- Peralta-Zaragoza, O.; Deas, J.; Meneses-Acosta, A.; De la O-Gómez, F.; Fernandez-Tilapa, G.; Gomez-Ceron, C.; Benitez-Boijseauneau, O.; Burguete-Garcia, A.; Torres-Poveda, K.; Bermudez-Morales, V.H.; et al. Relevance of miR-21 in regulation of tumor suppressor gene PTEN in human cervical cancer cells. BMC Cancer 2016, 16, 215.
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279.
- Boyd, J. Molecular biology in the clinicopathologic assessment of endometrial carcinoma subtypes. Gynecol. Oncol. 1996, 61, 163–165.
- Ramondetta, L.; Mills, G.B.; Burke, T.W.; Wolf, J.K. Adenovirus-mediated expression of p53 or p21 in a papillary serous endometrial carcinoma cell line (SPEC-2) results in both growth inhibition and apoptotic cell death: Potential application of gene therapy to endometrial cancer. Clin. Cancer Res. 2000, 6, 278–284.
- Ural, A.U.; Takebe, N.; Adhikari, D.; Ercikan-Abali, E.; Banerjee, D.; Barakat, R.; Bertino, J.R. Gene therapy for endometrial carcinoma with the herpes simplex thymidine kinase gene. Gynecol. Oncol. 2000, 76, 305–310.
- Grundker, C.; Huschmand Nia, A.; Emons, G. Gonadotropin-releasing hormone receptor-targeted gene therapy of gynecologic cancers. Mol. Cancer Ther. 2005, 4, 225–231.
- Xia, Y.; Li, X.; Sun, W. Applications of Recombinant Adenovirus-p53 Gene Therapy for Cancers in the Clinic in China. Curr. Gene Ther. 2020, 20, 127–141.
- Maxwell, G.L.; Chandramouli, G.V.; Dainty, L.; Litzi, T.J.; Berchuck, A.; Barrett, J.C.; Risinger, J.I. Microarray analysis of endometrial carcinomas and mixed mullerian tumors reveals distinct gene expression profiles associated with different histologic types of uterine cancer. Clin. Cancer Res. 2005, 11, 4056–4066.
- Maurice-Duelli, A.; Ndoye, A.; Bouali, S.; Leroux, A.; Merlin, J.L. Enhanced cell growth inhibition following PTEN nonviral gene transfer using polyethylenimine and photochemical internalization in endometrial cancer cells. Technol. Cancer Res. Treat. 2004, 3, 459–465.
- Banno, K.; Yanokura, M.; Kisu, I.; Yamagami, W.; Susumu, N.; Aoki, D. MicroRNAs in endometrial cancer. Int. J. Clin. Oncol. 2013, 18, 186–192.
- Donkers, H.; Bekkers, R.; Galaal, K. Diagnostic value of microRNA panel in endometrial cancer: A systematic review. Oncotarget 2020, 11, 2010–2023.
- Evans, P.; Brunsell, S. Uterine fibroid tumors: Diagnosis and treatment. Am. Fam. Physician 2007, 75, 1503–1508.
- Hassan, M.H.; Khatoon, N.; Curiel, D.T.; Hamada, F.M.; Arafa, H.M.; Al-Hendy, A. Toward gene therapy of uterine fibroids: Targeting modified adenovirus to human leiomyoma cells. Hum. Reprod. 2008, 23, 514–524.
- Niu, H.; Simari, R.D.; Zimmermann, E.M.; Christman, G.M. Nonviral vector-mediated thymidine kinase gene transfer and ganciclovir treatment in leiomyoma cells. Obstet. Gynecol. 1998, 91, 735–740.
- Al-Hendy, A.; Lee, E.J.; Wang, H.Q.; Copland, J.A. Gene therapy of uterine leiomyomas: Adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am. J. Obstet. Gynecol. 2004, 191, 1621–1631.
- Nair, S.; Curiel, D.T.; Rajaratnam, V.; Thota, C.; Al-Hendy, A. Targeting adenoviral vectors for enhanced gene therapy of uterine leiomyomas. Hum. Reprod. 2013, 28, 2398–2406.
- Abdelaziz, M.; Sherif, L.; ElKhiary, M.; Nair, S.; Shalaby, S.; Mohamed, S.; Eziba, N.; El-Lakany, M.; Curiel, D.; Ismail, N.; et al. Targeted Adenoviral Vector Demonstrates Enhanced Efficacy for In Vivo Gene Therapy of Uterine Leiomyoma. Reprod. Sci. 2016, 23, 464–474.
- Deng, Y.; Han, Q.; Mei, S.; Li, H.; Yang, F.; Wang, J.; Ge, S.; Jing, X.; Xu, H.; Zhang, T. Cyclin-dependent kinase subunit 2 overexpression promotes tumor progression and predicts poor prognosis in uterine leiomyosarcoma. Oncol. Lett. 2019, 18, 2845–2852.
- Li, Y.; Qiang, W.; Griffin, B.B.; Gao, T.; Chakravarti, D.; Bulun, S.; Kim, J.J.; Wei, J.J. HMGA2-mediated tumorigenesis through angiogenesis in leiomyoma. Fertil. Steril. 2020, 114, 1085–1096.
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799.
- Dabrosin, C.; Gyorffy, S.; Margetts, P.; Ross, C.; Gauldie, J. Therapeutic effect of angiostatin gene transfer in a murine model of endometriosis. Am. J. Pathol. 2002, 161, 909–918.
- Sun, J.J.; Yin, L.R.; Mi, R.R.; Ma, H.D.; Guo, S.J.; Shi, Y.; Gu, Y.J. Human endostatin antiangiogenic gene therapy mediated by recombinant adeno-associated virus vector in nude mouse with endometriosis. Zhonghua Fu Chan Ke Za Zhi 2010, 45, 45–50.
- Wang, N.; Liu, B.; Liang, L.; Wu, Y.; Xie, H.; Huang, J.; Guo, X.; Tan, J.; Zhan, X.; Liu, Y.; et al. Antiangiogenesis therapy of endometriosis using PAMAM as a gene vector in a noninvasive animal model. BioMed Res. Int. 2014, 2014, 546479.
- Othman, E.E.; Salama, S.; Ismail, N.; Al-Hendy, A. Toward gene therapy of endometriosis: Adenovirus-mediated delivery of dominant negative estrogen receptor genes inhibits cell proliferation, reduces cytokine production, and induces apoptosis of endometriotic cells. Fertil. Steril. 2007, 88, 462–471.
- Othman, E.E.; Zhu, Z.B.; Curiel, D.T.; Khatoon, N.; Salem, H.T.; Khalifa, E.A.; Al-Hendy, A. Toward gene therapy of endometriosis: Transductional and transcriptional targeting of adenoviral vectors to endometriosis cells. Am. J. Obstet. Gynecol. 2008, 199, 117.e1–117.e6.
- Koippallil Gopalakrishnan, A.R.; Pandit, H.; Metkari, S.M.; Warty, N.; Madan, T. Adenoviral vector encoding soluble Flt-1 engineered human endometrial mesenchymal stem cells effectively regress endometriotic lesions in NOD/SCID mice. Gene Ther. 2016, 23, 580–591.
- Senut, M.C.; Suhr, S.T.; Gage, F.H. Gene transfer to the rodent placenta in situ. A new strategy for delivering gene products to the fetus. J. Clin. Investig. 1998, 101, 1565–1571.
- Xing, A.; Boileau, P.; Cauzac, M.; Challier, J.C.; Girard, J.; Hauguel-de Mouzon, S. Comparative in vivo approaches for selective adenovirus-mediated gene delivery to the placenta. Hum. Gene Ther. 2000, 11, 167–177.
- Heikkila, A.; Hiltunen, M.O.; Turunen, M.P.; Keski-Nisula, L.; Turunen, A.M.; Rasanen, H.; Rissanen, T.T.; Kosma, V.M.; Manninen, H.; Heinonen, S.; et al. Angiographically guided utero-placental gene transfer in rabbits with adenoviruses, plasmid/liposomes and plasmid/polyethyleneimine complexes. Gene Ther. 2001, 8, 784–788.
- Bagot, C.N.; Troy, P.J.; Taylor, H.S. Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther. 2000, 7, 1378–1384.
- Taylor, H.S.; Arici, A.; Olive, D.; Igarashi, P. HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J. Clin. Investig. 1998, 101, 1379–1384.
- Luo, F.; Wen, Y.; Zhou, H.; Li, Z. Roles of long non-coding RNAs in cervical cancer. Life Sci. 2020, 256, 117981.
- Shi, Y.; He, R.; Yang, Y.; He, Y.; Shao, K.; Zhan, L.; Wei, B. Circular RNAs: Novel biomarkers for cervical, ovarian and endometrial cancer (Review). Oncol. Rep. 2020, 44, 1787–1798.
