You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Crispin Dass.
Chitosan is a linear polysaccharide composed of randomly distributed β-(1–4)-linked 2-amino-2-deoxy-d-glucopyranose (deacetylated units) and 2-acetamido-2-deoxy-d-glucopyranose (acetylated units). Chitosan is derived from chitin, a highly abundant natural biopolymer with a high cationic potential. Chitin is extracted from the exoskeleton of many living organisms, including crabs, shrimps, insects, and fungi.
- chitosan
- nanoparticle
- atherosclerosis
- cardiovascular
- drug
1. Atherosclerosis—A Brief Introduction
Atherosclerosis is a disease most typically characterised by thickening and/or hardening of the arteries caused by an accumulation of plaque in the lumen of an artery [1,2][1][2]. As such, the blood supply to the heart muscle, brain, or peripheral tissues may be compromised, which may be fatal if left unchecked [3,4,5][3][4][5]. Atherosclerosis is responsible for a majority of cardiovascular disease deaths [6,7][6][7]. Risk factors or triggers for the disease include high cholesterol, triglyceride and saturated fat levels (mainly through the diet), smoking, high blood pressure, obesity, and reduced or nil physical activity [8,9,10][8][9][10].
Of these, we thear most of the harmful effects were heard of cholesterol. It was Virchow, more than 100 years ago, that discovered that atheroma contained a yellow fat-containing matter, later to be identified as cholesterol by Windaus [11]. The current understanding of the development of atherosclerosis is the oxidation of lipids and lipoproteins that enhance the development of plaques [12]. The reader is directed to other excellent recent review articles that comprehensively describe the complex process of atherosclerosis development [13,14,15][13][14][15]. Apart from cholesterol, plaques are composed of fatty molecules such as phospholipids, calcium, and other constituents, remain stable throughout an individual’s life, or they become unstable and can grow to such a size that they pose a health risk from the stenosis (partial blockage of the artery), with a growing chance of disruption. Calcification of plaques may render the vessel rather incapable of regulating blood flow efficiently due to reduced flexibility within the vessel walls when compared to healthy patent vessels.
An atherosclerotic plaque can rupture suddenly and without much warning, leading to fatal events due to severe ischaemia [16,17][16][17]. While the good news is that the disease can be largely prevented through proper diet and activity in the early years of one’s life [18[18][19][20][21],19,20,21], once it sets in, it is quite hard to eradicate and often easy to exacerbate, even to the point of becoming severely life-threatening. Thus, newer methods for imaging (diagnosing) and managing (treating) this disease are being sought [22,23][22][23].
The disease process begins when low density lipoprotein (LDL; usually termed ‘bad cholesterol’) in the bloodstream passes through the gaps between endothelial cells and enters the intima of the arterial wall. The LDL particles, which are not harmful if they are left as is, unfortunately, are modified via oxidation into oxidized LDL (oxLDL). The immune response evoked by the oxidation of LDL cholesterol urges the endothelial cells near the inflammatory site to recruit monocytes from the bloodstream, which enter the intima (Figure 1). Once within the intima, the monocytes differentiate (or become specialised) into macrophages in the arterial wall and ingest the oxLDLs through surface-based scavenger receptors [24]. In a further step towards atherosclerosis, these macrophages convert to foam cells, lipid-laden phagocytic cells that contribute to the fatty streak architecture seen in diseased arterial walls. This sequesters cholesterol within the artery wall. The maturation of fatty streaks into more advanced plaques produces lesions that are usually covered with a fibrous cap composed of migrated smooth muscle cells (SMCs) and extracellular matrix (ECM) proteins such as collagen [25].
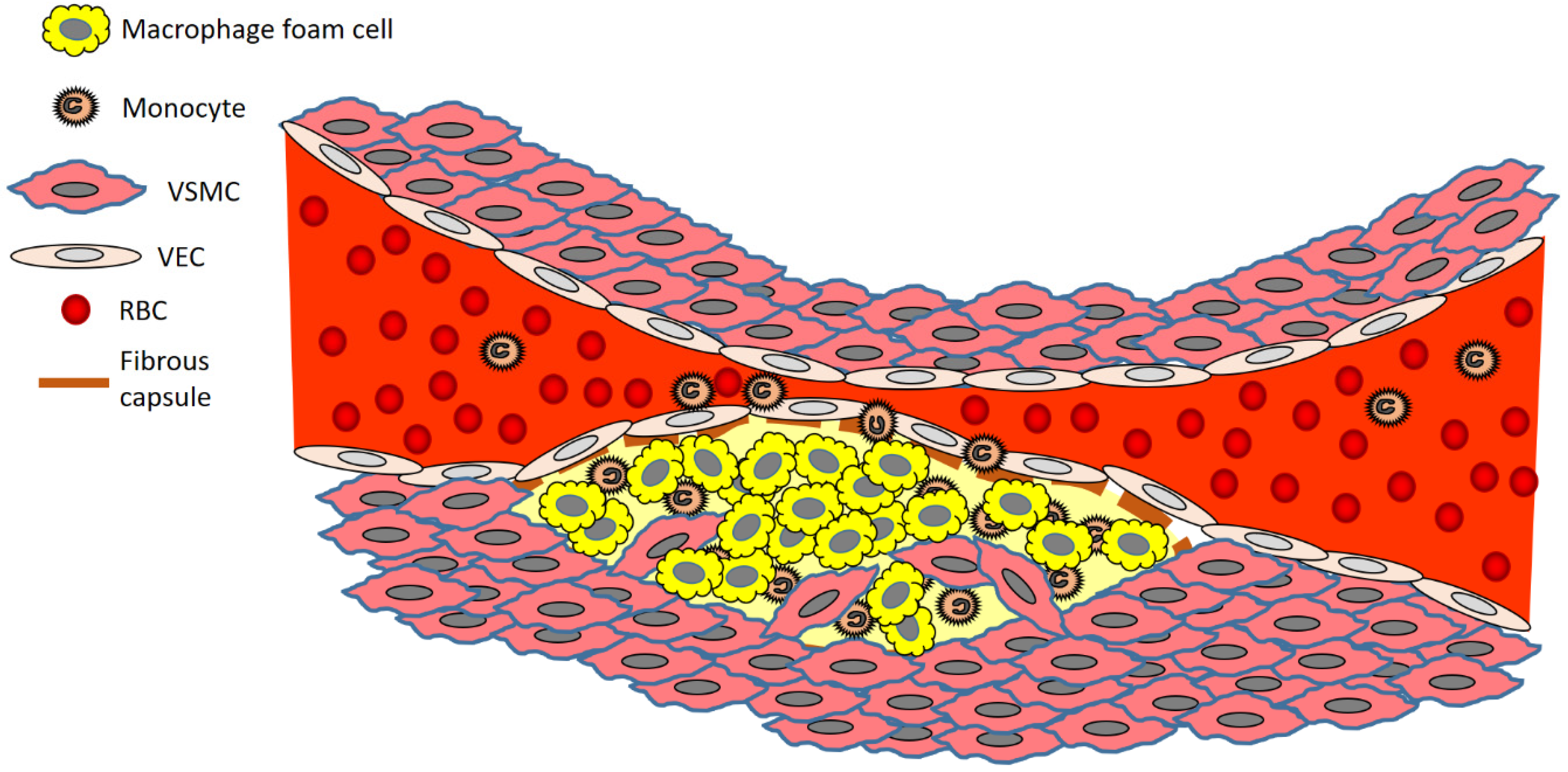
Figure 1. Brief overview of atherosclerosis development. Key: RBC, red blood cell; VEC, vascular endothelial cell; VSMC, vascular smooth muscle cell.
The migration of SMCs responds to a chemical signal produced during the accumulation of oxLDL, foam cells, and debris. This results in the formation of a fibrous cap, a layer of connective tissue that covers an atherosclerotic plaque and shields the lesion from the vascular lumen. The fibrous cap encloses a lipid-rich necrotic core composed of oxLDLs, cholesterol and apoptotic or necrotic cells that are unable to obtain sufficient nutrients for survival. As the inflammatory process progresses, apoptosis (cell death that occurs normally) and matrix degradation by matrix metalloproteinases (MMPs) become apparent. As inflammation escalates, accompanied by persistent foam cell recruitment and the ever more necrotic environment within the atherosclerotic plaque, further development and maturation of the atherosclerotic lesion ensue. This assists in the enlargement of the lipid-rich core and the thickening of the fibrous cap.
2. Chitosan—A Promising Biopolymer That Has Potential in Atherosclerotic Disease Management
Chitosan is a linear polysaccharide composed of randomly distributed β-(1–4)-linked 2-amino-2-deoxy-d-glucopyranose (deacetylated units) and 2-acetamido-2-deoxy-d-glucopyranose (acetylated units) [38][26]. Chitosan is derived from chitin, a highly abundant natural biopolymer with a high cationic potential [39][27]. Chitin is extracted from the exoskeleton of many living organisms, including crabs, shrimps, insects, and fungi. Chitosan possesses various highly desirable features, which include biocompatibility and versatility as far as formulation techniques are concerned. These characteristics make chitosan a highly sought after biomaterial with technological applications in cosmetics [40[28][29],41], pharmaceutical [42[30][31],43], and biomedical [44,45][32][33] industries. However, despite all this, as this reviewere will highlight, there is a deficiency of usage of chitosan in the formulation of nanoparticulate technologies that can be used for, and are being used for, atherosclerosis research (diagnostics, therapeutics, and theranostics). Of significant importance is the finding that chitosan per se possesses the ability to reduce cholesterol levels [46[34][35],47], which further adds to its arsenal as an attractive polymer in the development of drug delivery systems for the treatment of atherosclerosis. As chitosan has various beneficial effects on human health, for instance, in bone healing [48[36][37],49], it makes it quite an ideal biomaterial to employ in the drug delivery research sector. Various labs, including ours [50,51,52,53,54,55,56,57,58,59,60,61,62[38][39][40][41][42][43][44][45][46][47][48][49][50][51],63], have shown that chitosan is capable of encapsulating various types of therapeutic agents such as small molecule drugs to exploratory biologicals such as proteins and even DNA in delivery platforms of various sizes. As chitosan can even be ingested with no complications, this fact further cements the usefulness of this biopolymer in drug delivery R&D. Chitosan NPs have been formulated in the past using various methods, not limited to complex coacervation [64][52] and ionotropic gelation [65][53], both simple enough to carry out in most labs.3. Chitosan Nanoparticles Used in Atherosclerosis
Chitosan can be used as-is or as a chemically-modified form, such as a commonly used form in hydrophobically modified glycol chitosan (HGC). While not reviewed here, chitosan can also be derived into charged varieties, such as N-trimethyl chitosan chloride [66][54], which has been used, for instance, in diabetes [67][55] and cancer [68][56] research in drug delivery attempts. HGC NPs are biodegradable, have low immunogenicity and are adaptable to carry a variety of therapeutic agents in vivo [69,70][57][58]. Depending on the formulation technique used, this biopolymer can be relatively inexpensive and easy to use, as wthese [60,61,62,71,72,73][48][49][50][59][60][61] and others [74,75][62][63] have established previously. We now review tThe collection of reviewed articles in the literature that hahave used chitosan-based NPs in atherosclerosis research (none has yet been tested clinically, so the discussions revolve around cell culture and preclinical animal models testing)—that is, tested as a diagnostic, therapeutic or both diagnostic and therapeutic (theranostic) platform. One of the earliest studies, published in 2008 [76][64], was by a team who chemically conjugated an atherosclerotic plaque-homing peptide (‘AP peptide’, CRKRLDRNC) to HGC NPs. This peptide was derived from a process called biopanning—during which phage populations (from phage display libraries) are exposed to targets such as those found on VECs, macrophages and VSMCs that are found in atherosclerotic plaques, to selectively capture phages that bind to specific targets. As described in Park et al. [76][64], after several rounds of binding, washing, elution and amplification, the originally diverse phage population is reduced down to a phage population with a propensity to bind to the desired target. The authors used this technique as it had been before to identify differences in the molecular moieties expressed on the surfaces of VECs of differing tissues and even tumours [91,92][65][66]. The advantage of phage display technology is the easy discovery of peptides binding selectively to molecular targets, thus reducing the time and labour invested to generate lead candidates that have potential as imaging agents. Thus, tissue targeting, such as that to atherosclerotic plaques, may be possible by conjugating NPs to antibodies or peptides known to specifically bind to targets in specific disease tissues. Using bovine aortic endothelial cells (BAECs) as a model, Park et al. [76][64] were able to show both avid binding and more intracellular delivery of the NPs in these cells. The authors proposed that the activated BAECs may be expressing interleukin-4R (IL-4R) (which is induced by TNF-α exposure) on the surfaces of cells, suggesting that the AP peptide conjugated to HGC conjugate retained a binding affinity for IL-4R on activated BAECs. Thus, the NPs bound more selectively to TNF-α-activated BAECs than to unstimulated cells under both static and dynamic flow conditions. In vivo, the AP-tagged HGCCy5.5 nanoparticles bound better to atherosclerotic lesions in an LDL receptor-deficient (LDLr-/-) atherosclerotic mouse. NPs bound to the luminal surfaces of atherosclerotic lesions and inside the lesions, which are composed mainly of VSMCs and macrophages. While the authors expected that their AP-tagged HGC nanoparticles containing drugs would soon be developed for therapeutic purposes, no such further development has occurred to date, 13 years later. This seems to be the trend for other similar technologies discussed below, despite the relatively promising data obtained preclinically, some more than five years ago. Complex coacervation-prepared NPs composed of just chitosan and plasmid (expressing cholesteryl ester transfer protein, CETP) DNA were tested more than a decade ago [88][67]. As ourthe lab has demonstrated [50,55[38][43][44],56], this is one of the easiest methods to formulate NPs and depends essentially on the electrostatic attraction between the cationic chitosan and an anionic counterpart such as dextran sulphate [93,94][68][69] or oligonucleotides [95,96][70][71]. This is akin to the use of cationic liposomes to bind DNA [97][72], and one of the issues could be an inefficient release of the anionic DNA from the polycationic chitosan chains, a concern with other biopolymeric systems such as gelatin-based delivery systems [98][73]. In the Yuan et al. [88][67] study, the area of aortic lesions and intimal thickening were greatly reduced. One of the major advantages of an NP system such as this is the relative ease of manufacture, sometimes using simple instrumentation such as a speed-adjustable vortex mixer, with the added advantage of the use of cost-effective components and equipment that most labs would have inhouse. However, unlike other uses for gene delivery for other disease indications such as cancer, this technology has not really been further investigated or developed in atherosclerotic disease. It is important to point out, though, that the field of gene therapy has undergone several changes since the early clinical studies and showed side effects that even led to the death of some patients [99][74]. This is a far cry from the original promise the technology held [100[75][76],101], which has since lost some of its original allure. The intricacies of targeting a gene therapy construct such as plasmid DNA or viral vectors (such as adeno-associated vectors, AAVs) through the bloodstream to the target site, which is essentially part of that same bloodstream (that is a junction of the artery that is plagued with a lesion), is manifold. Not only does the delivery system have to overcome the mechanical difficulties of the blood flow, but also the intrinsic nature of its cargo (that is, DNA), which is naturally prone to degradation by nucleases In vivo. These, plus the fact that the cargo is sometimes 100 kDa in size, make this technology a difficult one to bring to fruition. Stabilin-2 is a glycoprotein highly expressed on macrophages, and VSMCs and VECs make atherosclerotic plaques [102][77]. Stabilin-2 acts as a receptor for advanced glycation end-products (AGEs) [103][78], chemical species that accumulate within atherosclerotic plaques of vessels. VECs and other cells constituting initial atherosclerotic events such as macrophages and foam cells of fatty streak lesions accumulate AGEs [104][79], which precede the development of maturing atherosclerotic plaques and then advanced lesions. In their study, Lee et al. [89][80] identified a peptide (CRTLTVRKC), termed S2P, from a phage display assay that was capable of binding avidly to stabilin-2. It took four rounds of selection to derive the peptide. S2P conjugated to HGC NPs was efficiently delivered to atherosclerotic plaques, becoming enriched in plaque regions. Interestingly, the work by this team was built on their findings reported in the same paper [89][80], which found that stabilin-2 was strongly expressed in VSMCs and VECs, as well as macrophages, of atherosclerotic lesions, suggesting that the protein could usefully serve as a target in atherosclerosis. Furthermore, the group established that injected S2P peptide homed to lymph nodes and spleen, that is, organs expressing stabilin-2, and colocalised with mouse stabilin-2 in the VECs of sinusoids within these tissues. Of slight concern was the finding that S2P localised strongly in the liver, which the authors attributed to the liver’s uptake of hydrophobic and low molecular-weight compounds. Epigallocatechin gallate (EGCG) is a polyphenolic compound found in green tea, which has vasculoprotective effects [105][81]. In humans, EGCG inhibits endothelial dysfunction and improves brachial artery dilation in patients with atherosclerosis [106][82]. However, it has poor stability and bioavailability in humans [107][83]. An attempt to nanoencapsulate EGCG in chitosan/polyaspartic acid NPs led to favourable stability In vitro (simulated gastric and intestinal fluids) and reduction of plaque load in vivo [77][84]. IHerein this study, the average ratios of lipid deposit area for EGCG NP-fed rabbits and EGCG-fed rabbits were 16.9 and 42.1, respectively, which showed that the EGCG NPs were significantly more effective against atherosclerosis compared with free EGCG. This was partially due to better protection of the phenolic compound within the NPs, given its low stability in water and physiological fluid because it readily undergoes oxidation, degradation, and polymerisation [108][85]. EGCG is unstable in sodium phosphate buffer (pH 7.4), where 80% of it is lost merely within 3 h [109,110][86][87]. In fact, the efficacy of EGCG NPs against rabbit atherosclerosis was close to that of simvastatin, a clinically used drug, which was in itself a remarkable achievement. Corresponding to these beneficial effects In vivo, the EGCG NP and EGCG dampened the levels of TG, TC, HDL-C, and LDL-C by 52, 55, 27, and 65% and 19, 26, 23, and 33%, respectively. Thus, the NPs showed efficacy in controlling levels of lipids that are detrimental in instigating and maintaining atherosclerosis. Bodyweight changes were not noted in the NP group, suggesting no gross toxic effects were evoked in treated mice. Superparamagnetic iron oxide NPs (SPIONs) formulated from a cationically derivatised chitosan were surface-modified with antibodies against either VCAM-1 or p-selectin [78][88]. In vitro studies confirmed the specific interaction of anti-VCAM-1 antibodies bound to the surface of SPIONs with aortic endothelial cells (AECs) derived from db/db mice, that is, cells represented those found in a state of inflammation typical of atherosclerotic plaques. Furthermore, these cells are usually inflamed as a complication of diabetes and, as mentioned above, constitute the early stages of plaque initiation before monocytes are attracted through VCAM-1 expression. In an ex vivo analysis, harvested aortic arch specimens with part of the descending aorta and brachiocephalic artery aortic ring present were obtained from ApoE/LDLR-/-mice with endothelial dysfunction and incubated with SPION-CCh-anti-VCAM-1 nanoparticles. The presence of SPIONs was confirmed by magnetic resonance imaging (MRI), and strong binding to the surface of the aorta was demonstrated. One finding of slight concern, and one that would need to be evaluated in an In vivo model in future, was that agglomerates of NPs were localised in the outer part of the aorta too, which may mean that the antibodies used are not sufficiently selective towards inflamed endothelium and may also interact with perivascular tissue. This could be well due to the non-selective binding of the SPIONs to the outer surface, perhaps due to the way the tissue was positioned during the assay period. Using a pegylated version of chitosan, Hirpara et al. [79][89] encapsulated a long-circulating form of NP ferrying rosuvastatin. Statins are 3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors proven to reduce total and LDL cholesterol [111][90]. In addition to the beneficial cholesterol-reducing effects, statins improve endothelial function, aid in the stabilisation of atherosclerotic plaques, and inhibit inflammatory and thrombogenic responses in arterial walls that enhance the risk of developing full-blown atherosclerosis [112][91]. To date, marketing surveillance has shown that long term statin therapy is generally well-tolerated, and this drug class is one of the frontline drugs used for atherosclerosis management clinically. In the study performed by Hirpara et al. [79][89], In vitro drug release demonstrated a sustained rate of release in phosphate buffer. In line with the pharmacokinetic (PK) study, in a hyperlipidaemic rat model, NPs enhanced the lipid-lowering capability of rosuvastatin compared to free drugs. The authors proposed that it was the PEGylation of NPs which helped to avoid opsonisation from the blood, allowing the NPs to circulate longer in the circulation, maintaining sustained release. Ferric-oxide-containing NPs made from chitosan, perfluorohexane (PFH), poly(lactic-co-glycolic acid) (PLGA), and dextran sulphate (DS) were tested in combination with low-intensity focused ultrasound (LIFU) irradiation to locate atherosclerotic lesions in mice [80][92]. These NPs carried ferric oxide, which could be picked up readily with MRI In vivo. In vitro, NPs selectively bound to activated macrophages via targeting class A scavenger receptors (SR-A) with the DS moiety. This was mirrored in an ex vivo aortic arch plaque model where NPs selectively bound to the lesion sites. In vivo, not only did the NPs target plaques, but they led to apoptosis of activated macrophages with the use of LIFU technology. The study built upon the expertise reported in an earlier paper by Zhu et al. [113][93], who successfully developed a novel NP that combined LIFU with an “explosion effect” within cells that was caused by an acoustic droplet vaporisation (ADV) phenomenon. In the process of ADV, a series of violent dynamic processes such as oscillation, expansion, contraction, and even the collapse of tiny bubbles in the liquid under the action of sound waves are generated, thereby inciting chemical reactions luminescence and subharmonics. These processes essentially damage the ultrastructure within cells, culminating in apoptosis and thereby death and removal of diseased cells [114][94]. For the past decade, nanotechnology-based RNAi therapeutic platforms have shown great promise for various disease applications, though not many such systems have been tested in the field of atherosclerosis research. A pegylated form of chitosan [81][95] was used to formulate NPs carrying mir-33, which is capable of reducing ATP binding cassette subfamily A member 1 (ABCA1). Ionic gelation mediated via tripolyphosphate (TPP) crosslinking, a relatively straightforward technique, was utilised for NP manufacture. In this technique, an ionic interaction between the cationic charge of primary amine groups of the chitosan with the anionic charge present in the nucleotides making up the miRNA mimics occurs, where TPP is used to stabilise the miRNA mimic–chitosan and chitosan–chitosan interactions. In vivo, a decreased reverse cholesterol transport (RCT) to the plasma, liver, and faeces was noted. Furthermore, when cholesterol efflux-promoting miRNAs were delivered with these NPs, ABCA1 expression and efflux into the RCT pathway were improved. In vitro, the efficiency of intracellular delivery of different formulations of these NPs to mouse macrophages was performed through an evaluation of the extent of cholesterol efflux to apolipoprotein A1 (ApoA1). NPs were also found to have no toxicity towards these cells in culture and were long-circulating owing to the pegylated moieties on their surface. Specifically, compared to empty NPs, mice injected with miR223-chNPs- or miR206-chNPs-loaded macrophages demonstrated increased plasma cholesterol (27% and 31% for miR223-chNPs, and 35% and 46% increase for miR206-chNPs over 24 and 48 h, respectively). Effluxed cholesterol uptake in the liver increased by 27% from miR223-chNPs and by 40% from miR206-chNPs-loaded macrophages. Treatment with miR206-chNPs also increased total faecal cholesterol excretion by 45% and 60% at 24 and 48 h, respectively, confirming that the NPs indeed were efficacious. CD47-targeted NPs were formulated in an attempt to deliver selectively to vascular endothelial cells (VECs) [82][96]. As the authors note, in atherosclerotic patients, high CD47 expression is commonly found in plaques. The nanoadjuvant approach employed by Yu et al. [82][96] relies on the fact that NP-encapsulated or -adsorbed antigens are the first targets of dendritic cell and macrophage phagocytosis and constitute an important step to achieving an effective immune response [115][97]. Nano-encapsulated antigens are abundant in antigen-presenting cells (macrophages, dendritic cells) and are strongly targeted to them. Furthermore, they are highly immunogenic. Fluorescently-labelled NPs were able to adsorb to VECs In vitro, though no attempt was made to examine whether NPs were, in fact, inside cells. In atherosclerosis-prone apolipoprotein E-deficient (ApoE-/-) mice, these NPs localised around the aortas, demonstrating the selective mode of delivery. Summarily, ithis study was established that atherosclerotic plaque clearance was promoted by blocking the expression of CD47 on the surface of atherosclerotic tissues by antibodies that were adsorbed on chitosan NPs. Additionally, these NPs, when combined with foam-like cells, exerted a negative immunomodulatory effect on exogenous immune cells, thereby further enhancing the inhibitory effect that was observed on atherosclerotic plaques in this study. In vivo, the chitosan-containing nanoadjuvants were shown to mediate the activation of type 1 regulatory (Tr1 or, more specifically, CD44þ Foxp3-Tr1) cells by exogenous foam cells. Tr1 cells inhibit NLRP3 ((NOD-, LRR- and pyrin domain-containing protein 3) activation in macrophages [116][98]. NLRP3-related inflammatory factors are highly expressed during the formation of microscopic plaques and are not only involved in early arterial plaque formation but are also in inflammatory responses and immune regulation In vivo [117,118][99][100]. Thus, a major inducer of atherosclerosis progression was regulated negatively. As a side issue, for most types of chitosan-containing NPs, these hyaluronic-acid (HA)-containing ones were both easy to manufacture and were non-toxic to the macrophage cells. As mentioned above, dysfunction at the endothelial layer leads to a build-up of the oxidized form of LDL in the intimal layer of the artery, whereby local inflammation culminates in the excess generation of reactive oxygen species, ROS [119][101]. ROS are ubiquitous in animals, given that they are byproducts of aerobic metabolism. As it we knowas known, oxygen’s outer valence shell consists of six electrons, resulting in two unpaired electrons. Thus, various ROS can be generated by increasing the electrons around the oxygen. Common examples include hydrogen peroxide (H2O2), superoxide anion (•O2−), peroxide (•O2−2), and hydroxyl radical (•OH) [120,121][102][103]. Macrophage membrane coated ROS-responsive NPs (MM-NPs) were formulated with oxidation-sensitive chitosan [83][104]. Using these coated NPs, this group was able to demonstrate results suggesting that these NPs may provide an effective strategy to escape macrophage clearance, reacting to ROS for enhancement of atorvastatin release. MM coating significantly increased NP circulation time by decreasing uptake by the reticuloendothelial system (RES). Most importantly, atorvastatin-carrying MM-coated NPs decreased plaque size at the aortic arch of ApoE-/-mice. They also stabilised plaques due to the increased presence of VSMCs and increased collagen.References
- Tîrziu, D.; Dobrian, A.; Tasca, C.; Simionescu, M.; Simionescu, N. Intimal thickenings of human aorta contain modified reassembled lipoproteins. Atherosclerosis 1995, 112, 101–114.
- Dass, C.R.; Jessup, W. Apolipoprotein A-I, Cyclodextrins and Liposomes as Potential Drugs for the Reversal of Atherosclerosis. A Review. J. Pharm. Pharmacol. 2000, 52, 731–761.
- Kessler, C.; Mitusch, R.; Guo, Y.; Rosengart, A.; Sheikhzadeh, A. Embolism from the aortic arch in patients with cerebral ischemia. Thromb. Res. 1996, 84, 145–155.
- Tulsyan, N.; Ouriel, K.; Kashyap, V. Emerging drugs in peripheral arterial disease. Expert Opin. Emerg. Drugs 2006, 11, 75–90.
- Reynolds, H.R. Myocardial infarction without obstructive coronary artery disease. Curr. Opin. Cardiol. 2012, 27, 655–660.
- Wrobel, T.P.; Mateuszuk, L.; Kostogrys, R.B.; Chlopicki, S.; Baranska, M. Quantification of plaque area and characterization of plaque biochemical composition with atherosclerosis progression in ApoE/LDLR(-/-) mice by FT-IR imaging. Analyst 2013, 138, 6645–6652.
- Saeedi, P.; Karuranga, S.; Hammond, L.; Kaundal, A.; Malanda, B.; Prystupiuk, M.; Matos, P. Cardiovascular diseases and risk factors knowledge and awareness in people with type 2 diabetes mellitus: A global evaluation. Diabetes Res. Clin. Pract. 2020, 165, 108194.
- Lechner, K.; von Schacky, C.; McKenzie, A.L.; Worm, N.; Nixdorff, U.; Lechner, B.; Kränkel, N.; Halle, M.; Krauss, R.M.; Scherr, J. Lifestyle factors and high-risk atherosclerosis: Pathways and mechanisms beyond traditional risk factors. Eur. J. Prev. Cardiol. 2020, 27, 394–406.
- Acosta, S.; Johansson, A.; Drake, I. Diet and Lifestyle Factors and Risk of Atherosclerotic Cardiovascular Disease—A Prospective Cohort Study. Nutrients 2021, 13, 3822.
- Sun, F.; Zhou, X.; Li, Q.; Li, Y.; Zhang, H.; Yan, Z.; He, H.; Zhao, Z.; Ke, Z.; Gao, Y.; et al. Achieving blood pressure targets and antihypertensive effects through metabolic surgery in type 2 diabetes patients with hypertension. Diabetes/Metab. Res. Rev. 2021, 37, e3422.
- Mayerl, C.; Lukasser, M.; Sedivy, R.; Niederegger, H.; Seiler, R.; Wick, G. Atherosclerosis research from past to present—On the track of two pathologists with opposing views, Carl von Rokitansky and Rudolf Virchow. Virchows Arch. 2006, 449, 96–103.
- Linton, M.F.; Yancey, P.G.; Davies, S.S.; Jerome, W.G.; Linton, E.F.; Song, W.L.; Doran, A.C.; Vickers, K.C. The role of lipids and lipoproteins in atherosclerosis. In Endotext ; MDText.com, Inc.: South Dartmouth, MA, USA, 2000; Available online: https://www.endotext.org/section/lipids (accessed on 25 December 2021).
- Boutagy, N.E.; Singh, A.K.; Sessa, W.C. Targeting the vasculature in cardiometabolic disease. J. Clin. Investig. 2022, 132, e148556.
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249.
- Eberhardt, N.; Giannarelli, C. How Single-Cell Technologies Have Provided New Insights into Atherosclerosis. Arter. Thromb. Vasc. Biol. 2022, 42, 243–252.
- Herem, J.W. Mural platelet microthrombi and major acute lesions of main epicardial arteries in sudden coronary death. Atherosclerosis 1974, 19, 529–541.
- Martin, L.R. Pathophysiology of myocardial infarction. Semin. Fam. Med. 1980, 1, 3–9.
- Blekkenhorst, L.C.; Sim, M.; Bondonno, C.P.; Bondonno, N.P.; Ward, N.C.; Prince, R.L.; Devine, A.; Lewis, J.R.; Hodgson, J.M. Cardiovascular Health Benefits of Specific Vegetable Types: A Narrative Review. Nutrients 2018, 10, 595.
- Hao, C.-L.; Lin, H.-L.; Ke, L.-Y.; Yen, H.-W.; Shen, K.-P. Pre-germinated brown rice extract ameliorates high-fat diet-induced metabolic syndrome. J. Food Biochem. 2019, 43, e12769.
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155.
- Gylling, H.; Strandberg, T.E.; Kovanen, P.T.; Simonen, P. Lowering Low-Density Lipoprotein Cholesterol Concentration with Plant Stanol Esters to Reduce the Risk of Atherosclerotic Cardiovascular Disease Events at a Population Level: A Critical Discussion. Nutrients 2020, 12, 2346.
- Zhang, Y.; Koradia, A.; Kamato, D.; Popat, A.; Little, P.J.; Ta, H.T. Treatment of atherosclerotic plaque: Perspectives on theranostics. J. Pharm. Pharmacol. 2019, 71, 1029–1043.
- Wang, Q.; Wang, Y.; Liu, S.; Sha, X.; Song, X.; Dai, Y.; Zhao, M.; Cai, L.; Xu, K.; Li, J. Theranostic nanoplatform to target macrophages enables the inhibition of atherosclerosis progression and fluorescence imaging of plaque in ApoE(−/−) mice. J. Nanobiotechnology 2021, 19, 222.
- Winckers, K.; Cate, H.T.; Hackeng, T.M. The role of tissue factor pathway inhibitor in atherosclerosis and arterial thrombosis. Blood Rev. 2013, 27, 119–132.
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arter. Thromb. Vasc. Biol. 2010, 30, 1282–1292.
- Tan, M.L.; Choong, P.F.; Dass, C.R. Cancer, chitosan nanoparticles and catalytic nucleic acids. J. Pharm. Pharmacol. 2009, 61, 3–12.
- Suwan, J.; Zhang, Z.; Li, B.; Vongchan, P.; Meepowpan, P.; Zhang, F.; Mousa, S.A.; Mousa, S.; Premanode, B.; Kongtawelert, P.; et al. Sulfonation of papain-treated chitosan and its mechanism for anticoagulant activity. Carbohydr. Res. 2009, 344, 1190–1196.
- Jo, H.-J.; Joo, S.-M.; Kim, J.Y.; Yu, K.-H.; Kim, S.W. Development of a Hybrid Chitosan- and Niacinamide-Coupled ZnO Nanoparticle Composite for Sun Protection Application. J. Nanomater. 2019, 2019, 5957606.
- Ta, Q.; Ting, J.; Harwood, S.; Browning, N.; Simm, A.; Ross, K.; Olier, I.; Al-Kassas, R. Chitosan nanoparticles for enhancing drugs and cosmetic components penetration through the skin. Eur. J. Pharm. Sci. 2021, 160, 105765.
- Wong, C.Y.; Martinez, J.; Al-Salami, H.; Dass, C.R. Quantification of BSA-loaded chitosan/oligonucleotide nanoparticles using reverse-phase high-performance liquid chromatography. Anal. Bioanal. Chem. 2018, 410, 6991–7006.
- Bhatt, H.; Bahadur, J.; Checker, R.; Ajgaonkar, P.; Vishwakarma, S.; Sen, D. Influence of molecular interactions on structure, controlled release and cytotoxicity of curcumin encapsulated chitosan—Silica nanostructured microspheres. Colloids Surf. B Biointerfaces 2021, 208, 112067.
- Thao, N.T.; Wijerathna, H.; Kumar, R.S.; Choi, D.; Dananjaya, S.; Attanayake, A. Preparation and characterization of succinyl chitosan and succinyl chitosan nanoparticle film: In Vitro and In Vivo evaluation of wound healing activity. Int. J. Biol. Macromol. 2021, 193, 1823–1834.
- Jaiswal, S.; Dutta, P.K.; Kumar, S.; Koh, J.; Lee, M.C.; Lim, J.W.; Pandey, S.; Garg, P. Synthesis, characterization and application of chitosan-N-(4-hydroxyphenyl)-methacrylamide derivative as a drug and gene carrier. Int. J. Biol. Macromol. 2022, 195, 75–85.
- Gallaher, C.M.; Munion, J.; Hesslink, R., Jr.; Wise, J.; Gallaher, D.D. Cholesterolreduction by glucomannan and chitosan is mediated by changes in cholesterol absorption and bile acid and fat excretion in rats. J. Nutr. 2000, 130, 2753–2759.
- Zhang, H.-L.; Tao, Y.; Guo, J.; Hu, Y.-M.; Su, Z.-Q. Hypolipidemic effects of chitosan nanoparticles in hyperlipidemia rats induced by high fat diet. Int. Immunopharmacol. 2011, 11, 457–461.
- Tan, M.L.; Shao, P.; Friedhuber, A.M.; van Moorst, M.; Elahy, M.; Indumathy, S.; Dunstan, D.; Wei, Y.; Dass, C.R. The potential role of free chitosan in bone trauma and bone cancer management. Biomaterials 2014, 35, 7828–7838.
- Shao, P.; Wei, Y.; Dass, C.R.; Zhang, G.; Wu, Z. Systemic Delivery of Free Chitosan Accelerates Femur Fracture Healing in Rats. Curr. Drug Targets 2018, 19, 460–466.
- Dass, C.R.; Contreras, K.G.; Dunstan, D.E.; Choong, P.F. Chitosan microparticles encapsulating PEDF plasmid demonstrate efficacy in an orthotopic metastatic model of osteosarcoma. Biomaterials 2007, 28, 3026–3033.
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Cafaggi, S.; Zibana, C.; Donalisio, M.; Cagno, V.; Lembo, D.; Caviglioli, G. Preparation, characterization and in vitro antiviral activity evaluation of foscarnet-chitosan nanoparticles. Colloids Surf. B Biointerfaces 2014, 118, 117–125.
- Russo, E.; Gaglianone, N.; Baldassari, S.; Parodi, B.; Croce, I.; Bassi, A.M.; Vernazza, S.; Caviglioli, G. Chitosan-clodronate nanoparticles loaded in poloxamer gel for intra-articular administration. Colloids Surf. B Biointerfaces 2016, 143, 88–96.
- Ta, H.T.; Dass, C.R.; Larson, I.; Choong, P.F.; Dunstan, D.E. A chitosan–dipotassium orthophosphate hydrogel for the delivery of Doxorubicin in the treatment of osteosarcoma. Biomaterials 2009, 30, 3605–3613.
- Ta, H.; Dass, C.R.; Larson, I.; Choong, P.F.; Dunstan, D.E. A chitosan hydrogel delivery system for osteosarcoma gene therapy with pigment epithelium-derived factor combined with chemotherapy. Biomaterials 2009, 30, 4815–4823.
- Tan, M.L.; Friedhuber, A.M.; Dunstan, D.E.; Choong, P.F.; Dass, C.R. The performance of doxorubicin encapsulated in chitosan–dextran sulphate microparticles in an osteosarcoma model. Biomaterials 2010, 31, 541–551.
- Tan, M.L.; Dunstan, D.E.; Friedhuber, A.M.; Choong, P.F.; Dass, C.R. A nanoparticulate system that enhances the efficacy of the tumoricide Dz13 when administered proximal to the lesion site. J. Control. Release 2010, 144, 196–202.
- Tan, M.L.; Friedhuber, A.M.; Dass, C.R. Co-nanoencapsulated doxorubicin and Dz13 control osteosarcoma progression in a murine model. J. Pharm. Pharmacol. 2013, 65, 35–43.
- Huanbutta, K.; Sangnim, T.; Cheewatanakornkool, K.; Sutthapitaksakul, L.; Thanawuth, K.; Sriamornsak, P. Physical stability of different chitosan salts in matrix tablet formulations. Pharm. Sci. Asia 2020, 47, 347–356.
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. The role of chitosan on oral delivery of peptide-loaded nanoparticle formulation. J. Drug Target. 2018, 26, 551–562.
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Formulation and characterisation of insulin-loaded chitosan nanoparticles capable of inducing glucose uptake in skeletal muscle cells in vitro. J. Drug Deliv. Sci. Technol. 2020, 57, 101738.
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Lyophilisation Improves Bioactivity and Stability of Insulin-Loaded Polymeric-Oligonucleotide Nanoparticles for Diabetes Treatment. AAPS PharmSciTech 2020, 21, 108.
- Wong, C.Y.; Martinez, J.; Zhao, J.; Al-Salami, H.; Dass, C.R. Development of orally administered insulin-loaded polymeric-oligonucleotide nanoparticles: Statistical optimization and physicochemical characterization. Drug Dev. Ind. Pharm. 2020, 46, 1238–1252.
- Wong CY, J.; Al-Salami, H.; Dass, C.R. β-Cyclodextrin-containing chitosan-oligonucleotide nanoparticles improve insulin bioactivity, gut cellular permeation and glucose consumption. J. Pharm. Pharmacol. 2021, 73, 726–739.
- Safarzadeh, M.; Mohammadi-Yeganeh, S.; Ghorbani-Bidkorbeh, F.; Haji Molla Hoseini, M. Chitosan based nanoformulation expressing miR-155 as a promising adjuvant to enhance Th1-biased immune responses. Life Sci. 2022, 297, 120459.
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan oligosaccharide/alginate nanoparticles as an effective carrier for astaxanthin with improving stability, in vitro oral bioaccessibility, and bioavailability. Food Hydrocoll. 2021, 124, 107246.
- Liu, H.; Ojha, B.; Morris, C.; Jiang, M.; Wojcikiewicz, E.P.; Rao, P.P.N.; Du, D. Positively Charged Chitosan and N-Trimethyl Chitosan Inhibit Aβ40 Fibrillogenesis. Biomacromolecules 2015, 16, 2363–2373.
- Sheng, J.; Han, L.; Qin, J.; Ru, G.; Li, R.; Wu, L.; Cui, D.; Yang, P.; He, Y.; Wang, J. N-Trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl. Mater. Interfaces 2015, 7, 15430–15441.
- Li, Z.; Li, X.; Cao, Z.; Xu, Y.; Lin, H.; Zhao, Y.; Wei, Y.; Qian, Z. Camptothecin nanocolloids based on N,N,N-trimethyl chitosan: Efficient suppression of growth of multiple myeloma in a murine model. Oncol. Rep. 2012, 27, 1035–1040.
- Park, J.H.; Kwon, S.; Nam, J.O.; Park, R.W.; Chung, H.; Seo, S.B.; Kim, I.S.; Kwon, I.C.; Jeong, S.Y. Self-assembled nanoparticles based on glycol chitosan bearing 5 -cholanic acid for RGD peptide delivery. J. Control. Release 2004, 95, 579–588.
- Kim, J.-H.; Kim, Y.-S.; Kim, S.; Park, J.H.; Kim, K.; Choi, K.; Chung, H.; Jeong, S.Y.; Park, R.-W.; Kim, I.-S.; et al. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release 2006, 111, 228–234.
- Dass, C.R.; Choong, P.F.M. Chitosan-mediated orally delivered nucleic acids: A gutful of gene therapy. J. Drug Target. 2008, 16, 257–261.
- Friedhuber, A.M.; Chandolu, V.; Manchun, S.; Donkor, O.; Sriamornsak, P.; Dass, C.R. Nucleotropic doxorubicin nanoparticles decrease cancer cell viability, destroy mitochondria, induce autophagy and enhance tumour necrosis. J. Pharm. Pharmacol. 2015, 67, 68–77.
- Lee, P.X.; Martinez, J.; Dass, C.R. Stimulation of bone regeneration with pigment epithelium-derived factor microparticles: Evidence in silico, in vitro and in vivo. Die Pharm.-Int. J. Pharm. Sci. 2016, 71, 382–389.
- Hecq, J.; Siepmann, F.; Siepmann, J.; Amighi, K.; Goole, J. Development and evaluation of chitosan and chitosan derivative nanoparticles containing insulin for oral administration. Drug Dev. Ind. Pharm. 2015, 41, 2037–2044.
- Kumar, S.; Kaur, P.; Bernela, M.; Rani, R.; Thakur, R. Ketoconazole encapsulated in chitosan-gellan gum nanocomplexes exhibits prolonged antifungal activity. Int. J. Biol. Macromol. 2016, 93, 988–994.
- Park, K.; Hong, H.-Y.; Moon, H.J.; Lee, B.-H.; Kim, I.-S.; Kwon, I.C.; Rhee, K. A new atherosclerotic lesion probe based on hydrophobically modified chitosan nanoparticles functionalized by the atherosclerotic plaque targeted peptides. J. Control. Release 2008, 128, 217–223.
- Hoogenboom, H.R. Designing and optimising library selection strategies for generating high-affinity antibodies. Trends Biotechnol. 1997, 15, 62–70.
- Kelly, K.A.; Nahrendorf, M.; Yu, A.M.; Reynolds, F.; Weissleder, R. In Vivo Phage Display Selection Yields Atherosclerotic Plaque Targeted Peptides for Imaging. Mol. Imaging Biol. 2006, 8, 201–207.
- Yuan, X.; Yang, X.; Cai, D.; Mao, D.; Wu, J.; Zong, L.; Liu, J. Intranasal immunization with chitosan/pCETP nanoparticles inhibits atherosclerosis in a rabbit model of atherosclerosis. Vaccine 2008, 26, 3727–3734.
- Chen, Y.; Mohanraj, V.J.; Parkin, J.E. Chitosan-dextran sulfate nanoparticles for delivery of an anti-angiogenesis peptide. Lett. Pept. Sci. 2003, 10, 621–629.
- Chaiyasan, W.; Srinivas, S.P.; Tiyaboonchai, W. Mucoadhesive Chitosan–Dextran Sulfate Nanoparticles for Sustained Drug Delivery to the Ocular Surface. J. Ocul. Pharmacol. Ther. 2013, 29, 200–207.
- Bozka, A.; Saka, O.M. Chitosan-DNA nanoparticles: Effect on DNA integrity, bacterial transformation and transfection efficiency. J. Drug Target. 2004, 12, 281–288.
- Huang, T.; Song, X.; Jing, J.; Zhao, K.; Shen, Y.; Zhang, X.; Yue, B. Chitosan-DNA nanoparticles enhanced the immunogenicity of multivalent DNA vaccination on mice against Trueperella pyogenes infection. J. Nanobiotechnol. 2018, 16, 8.
- Dass, C.R. Biochemical and biophysical characteristics of lipoplexes pertinent to solid tumour gene therapy. Int. J. Pharm. 2002, 241, 1–25.
- Nezhadi, S.H.; Choong, P.; Lotfipour, F.; Dass, C.R. Gelatin-based delivery systems for cancer gene therapy. J. Drug Target. 2009, 17, 731–738.
- Carmen, I.H. A Death in the Laboratory: The Politics of the Gelsinger Aftermath. Mol. Ther. 2001, 3, 425–428.
- Dass, C.R.; Walker, T.L.; Decruz, E.E.; Burton, M.A. Cationic Liposomes and Gene Therapy for Solid Tumors. Drug Deliv. 1997, 4, 151–165.
- Anderson, W.F. Human gene therapy. Nature 1998, 392, 25–30.
- Park, S.-Y.; Jung, M.-Y.; Kim, H.-J.; Lee, S.-J.; Kim, S.-Y.; Lee, B.-H.; Kwon, T.-H.; Park, R.-W.; Kim, I.-S. Rapid cell corpse clearance by stabilin-2, a membrane phosphatidylserine receptor. Cell Death Differ. 2007, 15, 192–201.
- Tamura, Y.; Adachi, H.; Osuga, J.-I.; Ohashi, K.; Yahagi, N.; Sekiya, M.; Okazaki, H.; Tomita, S.; Iizuka, Y.; Shimano, H.; et al. FEEL-1 and FEEL-2 Are Endocytic Receptors for Advanced Glycation End Products. J. Biol. Chem. 2003, 278, 12613–12617.
- Nakamura, Y.; Horii, Y.; Nishino, T.; Shiiki, H.; Sakaguchi, Y.; Kagoshima, T.; Dohi, K.; Makita, Z.; Vlassara, H.; Bucala, R. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am. J. Pathol. 1993, 143, 1649–1656.
- Lee, G.Y.; Kim, J.-H.; Oh, G.T.; Lee, B.-H.; Kwon, I.C.; Kim, I.-S. Molecular targeting of atherosclerotic plaques by a stabilin-2-specific peptide ligand. J. Control. Release 2011, 155, 211–217.
- Benlloch, M.; Cuerda-Ballester, M.; Drehmer, E.; Platero, J.L.; Carrera-Juliá, S.; López-Rodríguez, M.M.; Ceron, J.J.; Tvarijonaviciute, A.; Navarro, M.; Moreno, M.L.; et al. Possible Reduction of Cardiac Risk after Supplementation with Epigallocatechin Gallate and Increase of Ketone Bodies in the Blood in Patients with Multiple Sclerosis. A Pilot Study. Nutrients 2020, 12, 3792.
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic Study of Catechin Stability: Effects of pH, Concentration, and Temperature. J. Agric. Food Chem. 2012, 60, 12531–12539.
- Lee, M.-J.; Maliakal, P.; Chen, L.; Meng, X.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (-)-epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032.
- Hong, Z.; Xu, Y.; Yin, J.-F.; Jin, J.; Jiang, Y.; Du, Q. Improving the Effectiveness of (−)-Epigallocatechin Gallate (EGCG) against Rabbit Atherosclerosis by EGCG-Loaded Nanoparticles Prepared from Chitosan and Polyaspartic Acid. J. Agric. Food Chem. 2014, 62, 12603–12609.
- Lambert, J.D.; Lee, M.-J.; Diamond, L.; Ju, J.; Hong, J.; Bose, M.; Newmark, H.L.; Yang, C.S. Dose-dependent levels of epigallocatechin-3-gallate in human colon cancer cells and mouse plasma and tissues. Drug Metab. Dispos. 2006, 34, 8–11.
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. J. Agric. Food Chem. 2005, 53, 9478–9484.
- Krook, M.A.; Hagerman, A.E. Stability of polyphenols epigallocatechin gallate and pentagalloyl glucose in a simulated digestive system. Food Res. Int. 2012, 49, 112–116.
- Kaczyńska, A.; Guzdek, K.; Derszniak, K.; Karewicz, A.; Lewandowska-Łańcucka, J.; Mateuszuk, Ł.; Skórka, T.; Banasik, T.; Jasiński, K.; Kapusta, C.; et al. Novel nanostructural contrast for magnetic resonance imaging of endothelial inflammation: Targeting SPIONs to vascular endothelium. RSC Adv. 2016, 6, 72586–72595.
- Hirpara, M.R.; Manikkath, J.; Sivakumar, K.; Managuli, R.S.; Gourishetti, K.; Krishnadas, N.; Shenoy, R.R.; Jayaprakash, B.; Rao, C.M.; Mutalik, S. Long circulating PEGylated-chitosan nanoparticles of rosuvastatin calcium: Development and in vitro and in vivo evaluations. Int. J. Biol. Macromol. 2018, 107, 2190–2200.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22.
- Colhoun, H.M.; Betteridge, D.J.; Durrington, P.N.; Hitman, G.A.; Neil, H.A.; Livingstone, S.J.; Thomason, M.J.; Mackness, M.I.; Charlton-Menys, V.; Fuller, J.H.; et al. Primary prevention of cardiovascular disease with atorvastatinin type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): Multicentre randomized placebo-controlled trial. Lancet 2004, 364, 685–696.
- Ye, M.; Zhou, J.; Zhong, Y.; Xu, J.; Hou, J.; Wang, X.; Wang, Z.-G.; Guo, D. SR-A-Targeted Phase-Transition Nanoparticles for the Detection and Treatment of Atherosclerotic Vulnerable Plaques. ACS Appl. Mater. Interfaces 2019, 11, 9702–9715.
- Zhu, L.; Zhao, H.; Zhou, Z.; Xia, Y.; Wang, Z.; Ran, H.; Li, P.; Ren, J. Peptide-Functionalized Phase-Transformation Nanoparticles for Low Intensity Focused Ultrasound-Assisted Tumor Imaging and Therapy. Nano Lett. 2018, 18, 1831–1841.
- Ho, Y.-J.; Chang, Y.-C.; Yeh, C.-K. Improving Nanoparticle Penetration in Tumors by Vascular Disruption with Acoustic Droplet Vaporization. Theranostics 2015, 6, 392–403.
- Nguyen, M.-A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.-C.; Cottee, M.L.; Afolayan, E.; Farah, E.; Kahiel, Z.; et al. Delivery of MicroRNAs by Chitosan Nanoparticles to Functionally Alter Macrophage Cholesterol Efflux in vitro and in vivo. ACS Nano 2019, 13, 6491–6505.
- Yu, J.; Ruan, Q.; Nie, X. Synthesis and characterization of atherosclerotic target anti-CD47 functionalized by nano- polyelectrolyte complexes between chitosan and hyaluronic acid and in vivo and in vitro targeting experiments. Adv. Clin. Exp. Med. 2020, 29, 1407–1415.
- Cybulsky, M.I.; Cheong, C.; Robbins, C.S. Macrophages and dendritic cells: Partners in atherogenesis. Circ. Res. 2016, 118, 637–652.
- Yao, Y.; Vent-Schmidt, J.; McGeough, M.D.; Wong, M.; Hoffman, H.M.; Steiner, T.S.; Levings, M.K. Tr1 cells, but not Foxp3þ regulatory T cells, suppress NLRP3 inflammasome activation via an IL-10-dependent mechanism. J. Immunol. 2015, 195, 488–497.
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361.
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.-J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203.
- Allahverdian, S.; Chehroudi, A.C.; McManus, B.M.; Abraham, T.; Francis, G.A. Contribution of Intimal Smooth Muscle Cells to Cholesterol Accumulation and Macrophage-Like Cells in Human Atherosclerosis. Circulation 2014, 129, 1551–1559.
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344.
- Bardaweel, S.K.; Gul, M.; Alzweiri, M.; Ishaqat, A.; ALSalamat, H.A.; Bashatwah, R.M. Reactive Oxygen Species: The Dual Role in Physiological and Pathological Conditions of the Human Body. Eurasian J. Med. 2018, 50, 193–201.
- Gao, C.; Huang, Q.; Liu, C.; Kwong, C.H.T.; Yue, L.; Wan, J.-B.; Lee, S.M.Y.; Wang, R. Treatment of atherosclerosis by macrophage-biomimetic nanoparticles via targeted pharmacotherapy and sequestration of proinflammatory cytokines. Nat. Commun. 2020, 11, 2622.
More
