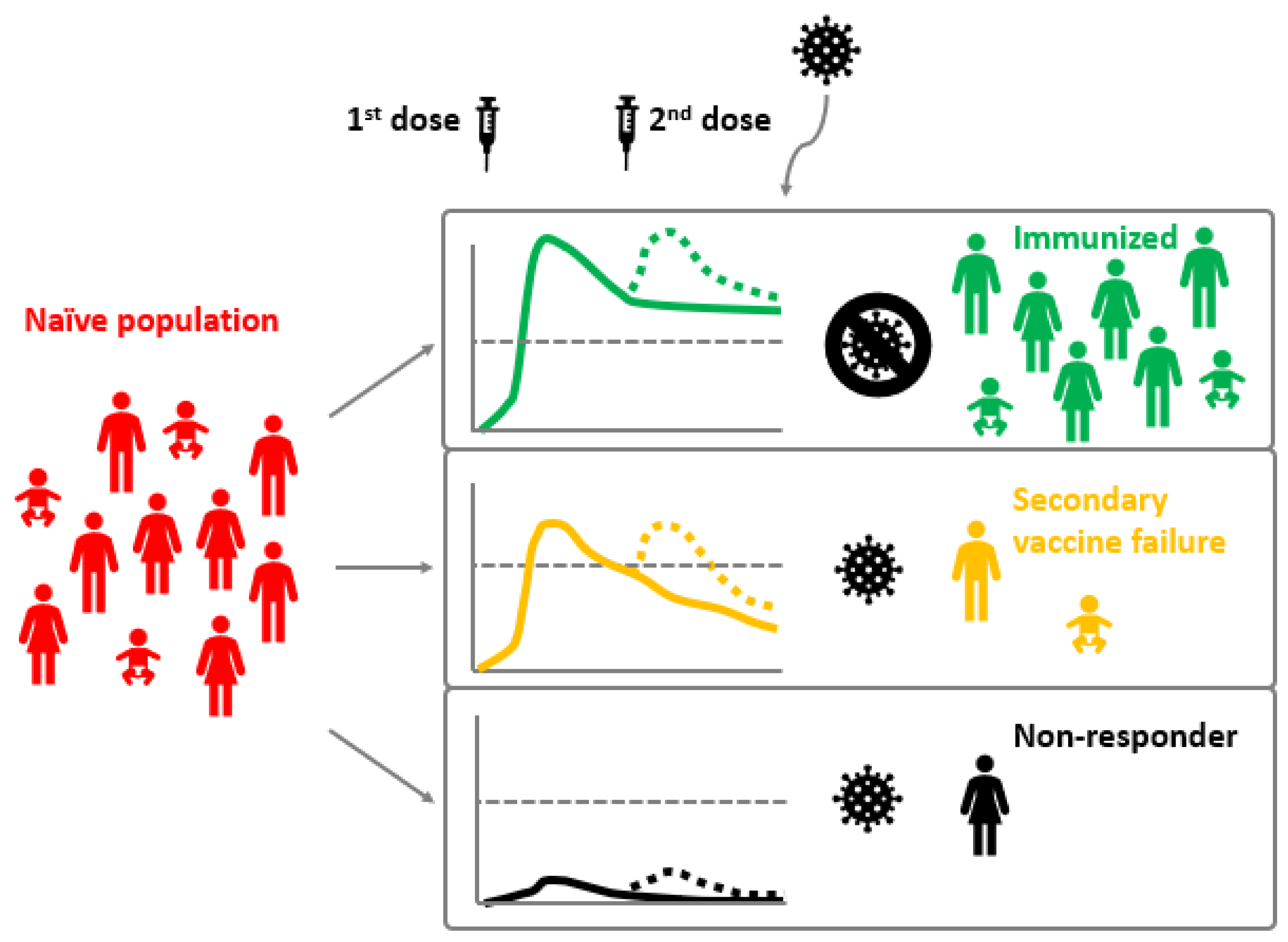
Measles is one of the most contagious diseases known to man. Despite the existence of a safe and effective live attenuated vaccine, measles can appear in vaccinated individuals. Paradoxically, breakthrough cases increase as vaccination coverage in the general population rises. In measles endemic areas, breakthrough cases represent less than 10% of total infections, while in areas with high vaccination coverage these are over 10% of the total. Two different vaccination failures have been described: primary vaccination failure, which consists in the complete absence of humoral response and occurs in around 5% of vaccinated individuals; and secondary vaccination failure is due to waning immunity or incomplete immunity and occurs in 2–10% of vaccinees. Vaccination failures are generally associated with lower viral loads and milder disease (modified measles) since vaccination limits the risk of complicated disease. Vaccination failure seems to occur between six and twenty-six years after the last vaccine dose administration.
- measles
- breakthrough infection
- measles vaccine
- vaccination failures
1. Introduction
2. Measles Vaccines and Immunization Programs
Several attenuated measles vaccines are available worldwide, either as single-virus vaccines or in combination with the rubella and mumps vaccines (MMR) or with the rubella, mumps, and chickenpox vaccines (MMRV). Although there are 24 recognized MV genotypes, MV is considered serologically monotypic [7] and most of the available vaccine strains derive from the Edmonston strain, isolated in 1954 [8]. The principal target of human antibodies is the H protein. Sequence analyses of the H gene performed in several studies did not show specific mutations associated with immune escaping, and this antigenic stability could be at the basis of the effectiveness of the present vaccine [9][10][11][12]. Likewise, no significant differences have been found in strains circulating in vaccinated or not vaccinated individuals [13][14]. The measles vaccine induces both humoral and cellular immune responses and antibodies appear between 12 and 15 days after vaccination, peaking at 21 to 28 days [2]. Measles immunization programs consist of the administration of two doses of measles vaccines. Although there are variations between vaccination calendars of the various countries, according to WHO recommendations for routine immunization, the first dose is usually given at the age of approximately nine months in countries with ongoing measles transmission, in which the risk of measles mortality remains high, and, to take advantage of the higher seroconversion rates achieved at an older age, at the age of 12 months in countries with low levels of measles transmission. The second dose should be administered at 15 to 18 months of age or at school entry [15][16]. However, every opportunity should be taken to vaccinate all children that missed one or both routine doses [16].3. Measles Virus Infection and Vaccine Failure
Despite the availability of a safe and highly effective live attenuated vaccine, measles can manifest in individuals with a documented vaccination history. During the Cincinnati and St. Louis epidemics of 1971–1973, Cherry et al., and Plotkin et al., described for the first time the existence of measles vaccination failure cases [17][18][19]. Indeed, nowadays it has been observed that the duration of protection conferred by measles vaccine is more variable and shorter than that acquired through measles infection, with an estimated 5% of children losing protective antibody titres 10–15 years after vaccination [2][14][20]. Breakthrough cases, which occur when a person becomes sick with a disease despite having received the vaccine for that disease (vaccine failure), do play an important role in the epidemiology of the disease. As vaccination coverage increases in the general population, a proportional increase in the frequency of measles cases among vaccinated individuals is expected, as long as MV circulates [21]. This happens because, with fewer non-vaccinated individuals, most of the susceptible subjects are those that did not develop a protective immune response after vaccination (Figure 1). Indeed, the portion of breakthrough cases over the total of measles infections is higher in countries with high vaccination coverage, as described in various studies [22][23][24][25].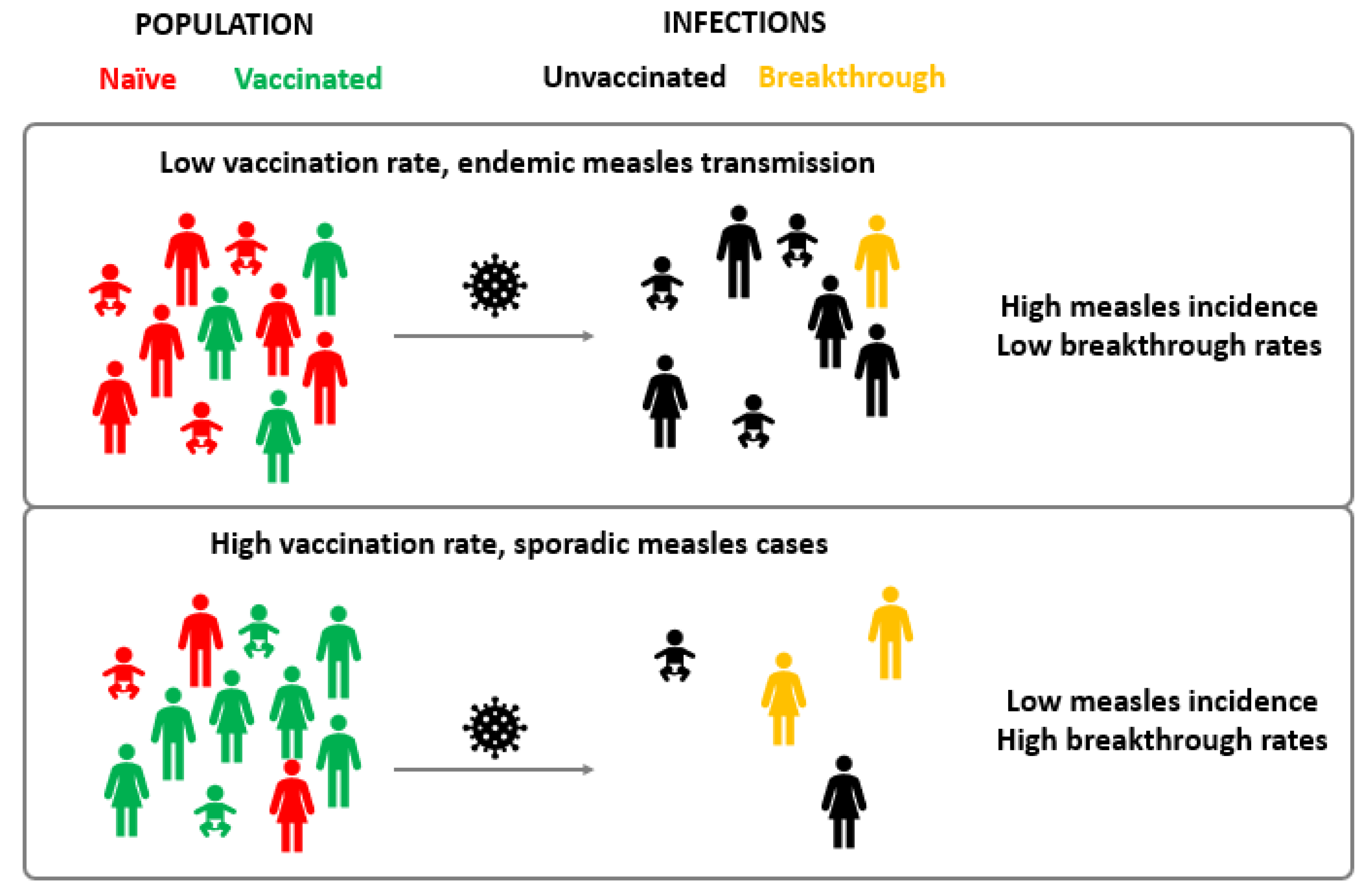
4. Vaccine Failure Classification
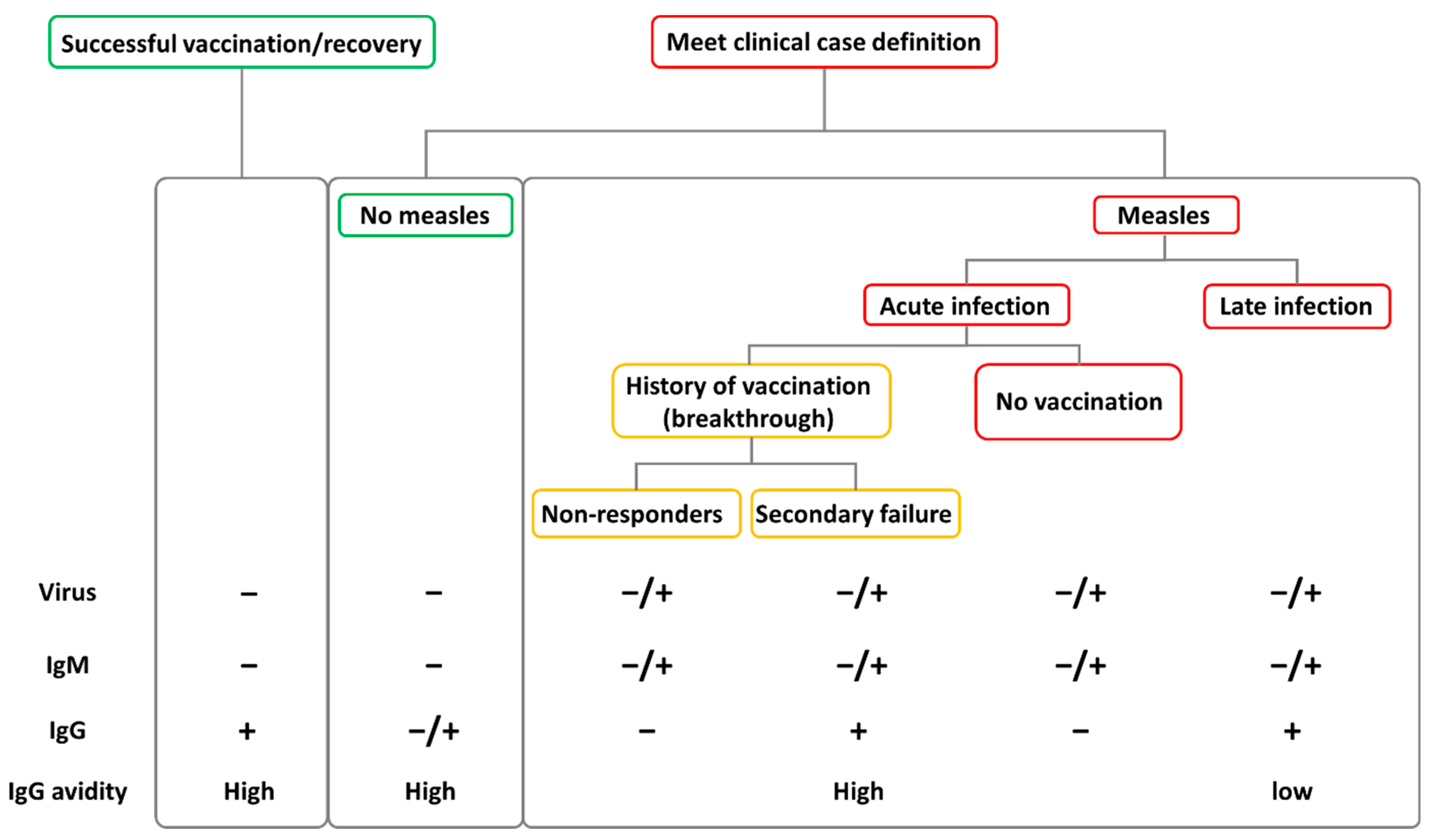
5. Clinical Manifestations of Breakthrough Cases and Diagnostic Challenges
6. Onward Transmission from Breakthrough Cases
Although rare, transmission from vaccine failure cases is possible, with vaccinated people acting both as index cases as well as secondary transmitters. Onward transmission from vaccinated cases to susceptible individuals seems to be limited to specific settings where close contacts are more common, such as familiar or nosocomial environments. The low rate of transmission from breakthrough cases may be associated with the elevated and rapid production of neutralizing antibodies that quickly reduce the viral load, but also with the manifestation with milder symptoms (i.e., mild, or unproductive cough) that reduces the likelihood of an effective transmission of the virus [32]. Nevertheless, the fact that a MV infection and onward transmission can both occur for subjects with vaccine failures, underscores the need to maintain a high index of suspicion for measles during an outbreak and to monitor all subjects despite (presumed) prior vaccination or disease [36]. Specifically, the same effort in tracing contacts should be dedicated to identifying possible infections amongst vaccinated and unvaccinated subjects and vaccinated cases should also carefully follow virus containment procedures. This is of particular importance in health care settings.
7. Booster Doses and Catch-Up Vaccination
Breakthrough infections occur between six and twenty-six years after the last measles vaccination [13][14][22][23][37][38]. A progressive decrease in levels of anti-MV antibodies as time since vaccination increases has been observed, as also shown by a prospective cohort study performed in the United States [20]. LeBaron and colleagues, in a study conducted in schoolchildren in a post-elimination environment, observed a decrease in neutralizing antibodies ten years after vaccination, with 4.7% of fully vaccinated children considered potentially susceptible to reinfection (neutralizing titres lower than 120 mIU/mL) [20]. The analysis conducted by Pacenti et al., showed that in over 90% of subjects, antibody titres remained above the level of protection up to 30 years after vaccination [14]. These results were consistent with previous studies [39]. Therefore, even if the percentage of susceptible individuals several years after vaccination seems to be low, population immunity to MV should be monitored, especially in adult age groups, to assess potential declines of protection. Catch-up vaccination is recommended for all individuals (children, adolescents, and adults) who have not received the first or second dose of MV vaccine. However, in many European countries catch-up vaccination programs are not efficiently conducted or well accepted. Consequently, measles outbreaks are still occurring despite significantly increasing vaccination rates, with many adolescents and young adults (up to 40 years of age) being affected [40]. Furthermore, during 2020, childhood immunization services have been disrupted by the COVID-19 pandemic in about 70 countries, with around 80 million children being affected [41]. Reduced routine vaccination coverage without catch-up vaccination may lead to an increase in measles burden worldwide. The effect of lower measles vaccine coverage has not yet resulted in an increase in the number of cases and deaths, probably because prevention and control measures introduced to reduce the spread of SARS-CoV-2 have also reduced the spread of MV [42]. Nonetheless, it is crucial to implement catch-up vaccination campaigns and close this immunization gap before the consequences of the reduced coverage will start manifesting.References
- Measles. Available online: https://www.who.int/news-room/fact-sheets/detail/measles (accessed on 21 May 2022).
- Moss, W.J.; Griffin, D.E. Global Measles Elimination. Nat. Rev. Microbiol. 2006, 4, 900–908.
- Rima, B.; Balkema-Buschmann, A.; Dundon, W.G.; Duprex, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.; Lee, B.; Rota, P.; et al. ICTV Virus Taxonomy Profile: Paramyxoviridae. J. Gen. Virol. 2019, 100, 1593–1594.
- World Health Organization. Manual for the Laboratory Diagnosis of Measles and Rubella Virus Infection; WHO/IVB/07.01; World Health Organization: Geneva, Switzerland, 2007.
- Minnich, L.L.; Goodenough, F.; Ray, C.G. Use of Immunofluorescence to Identify Measles Virus Infections. J. Clin. Microbiol. 1991, 29, 1148–1150.
- World Health Organization. Guidelines for Measles and Rubella Outbreak Investigation and Response in the WHO European Region; World Health Organization: Geneva, Switzerland, 2013; p. 39.
- Hashiguchi, T.; Maenaka, K.; Yanagi, Y. Measles Virus Hemagglutinin: Structural Insights into Cell Entry and Measles Vaccine. Front. Microbiol. 2011, 2, 247.
- Baldo, A.; Galanis, E.; Tangy, F.; Herman, P. Biosafety Considerations for Attenuated Measles Virus Vectors Used in Virotherapy and Vaccination. Hum. Vaccines Immunother. 2016, 12, 1102–1116.
- Ciceri, G.; Canuti, M.; Bianchi, S.; Gori, M.; Piralla, A.; Colzani, D.; Libretti, M.; Frati, E.R.; Baggieri, M.; Lai, A.; et al. Genetic Variability of the Measles Virus Hemagglutinin Gene in B3 Genotype Strains Circulating in Northern Italy. Infect. Genet. Evol. 2019, 75, 103943.
- Woelk, C.H.; Jin, L.; Holmes, E.C.; Brown, D.W.G.Y. Immune and Artificial Selection in the Haemagglutinin (H) Glycoprotein of Measles Virus. J. Gen. Virol. 2001, 82, 2463–2474.
- Xu, S.; Zhang, Y.; Zhu, Z.; Liu, C.; Mao, N.; Ji, Y.; Wang, H.; Jiang, X.; Li, C.; Tang, W.; et al. Genetic Characterization of the Hemagglutinin Genes of Wild-Type Measles Virus Circulating in China, 1993–2009. PLoS ONE 2013, 8, e73374.
- Bianchi, S.; Canuti, M.; Ciceri, G.; Gori, M.; Colzani, D.; Dura, M.; Pennati, B.M.; Baggieri, M.; Magurano, F.; Tanzi, E.; et al. Molecular Epidemiology of B3 and D8 Measles Viruses through Hemagglutinin Phylogenetic History. Int. J. Mol. Sci. 2020, 21, 4435.
- Bianchi, S.; Gori, M.; Fappani, C.; Ciceri, G.; Canuti, M.; Colzani, D.; Dura, M.; Terraneo, M.; Lamberti, A.; Baggieri, M.; et al. Characterization of Vaccine Breakthrough Cases during Measles Outbreaks in Milan and Surrounding Areas, Italy, 2017–2021. Viruses 2022, 14, 1068.
- Pacenti, M.; Maione, N.; Lavezzo, E.; Franchin, E.; Dal Bello, F.; Gottardello, L.; Barzon, L. Measles Virus Infection and Immunity in a Suboptimal Vaccination Coverage Setting. Vaccines 2019, 7, 199.
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502.
- WHO Recommendations for Routine Immunization—Summary Tables. Available online: https://www.who.int/teams/immunization-vaccines-and-biologicals/policies/who-recommendations-for-routine-immunization---summary-tables (accessed on 12 June 2022).
- Cherry, J.D.; Feigin, R.D.; Lobes, L.A.; Hinthorn, D.R.; Shackelford, P.G.; Shirley, R.H.; Lins, R.D.; Choi, S.C. Urban Measles in the Vaccine Era: A Clinical, Epidemiologic, and Serologic Study. J. Pediatr. 1972, 81, 217–230.
- Cherry, J.D.; Feigin, R.D.; Shackelford, P.G.; Hinthorn, D.R.; Schmidt, R.R. A Clinical and Serologic Study of 103 Children with Measles Vaccine Failure. J. Pediatr. 1973, 82, 802–808.
- Plotkin, S.A. Failures of Protection by Measles Vaccine. J. Pediatr. 1973, 82, 908–911.
- LeBaron, C.W.; Beeler, J.; Sullivan, B.J.; Forghani, B.; Bi, D.; Beck, C.; Audet, S.; Gargiullo, P. Persistence of Measles Antibodies after 2 Doses of Measles Vaccine in a Postelimination Environment. Arch. Pediatr. Adolesc. Med. 2007, 161, 294–301.
- Arima, Y.; Oishi, K. Letter to the Editor: Measles Cases among Fully Vaccinated Persons. Eurosurveillance 2018, 23, 1800449.
- Cherry, J.D.; Zahn, M. Clinical Characteristics of Measles in Previously Vaccinated and Unvaccinated Patients in California. Clin. Infect. Dis. 2018, 67, 1315–1319.
- López-Perea, N.; Fernández-García, A.; Echevarría, J.E.; de Ory, F.; Pérez-Olmeda, M.; Masa-Calles, J. Measles in Vaccinated People: Epidemiology and Challenges in Surveillance and Diagnosis in the Post-Elimination Phase. Spain, 2014–2020. Viruses 2021, 13, 1982.
- Risco-Risco, C.; Masa-Calles, J.; López-Perea, N.; Echevarría, J.E.; Rodríguez-Caravaca, G. Epidemiology of Measles in Vaccinated People, Spain 2003-2014. Enferm. Infect. Microbiol. Clin. 2017, 35, 569–573.
- Sundell, N.; Dotevall, L.; Sansone, M.; Andersson, M.; Lindh, M.; Wahlberg, T.; Tyrberg, T.; Westin, J.; Liljeqvist, J.-Å.; Bergström, T.; et al. Measles Outbreak in Gothenburg Urban Area, Sweden, 2017 to 2018: Low Viral Load in Breakthrough Infections. Eurosurveillance 2019, 24, 1900114.
- Wiedermann, U.; Garner-Spitzer, E.; Wagner, A. Primary Vaccine Failure to Routine Vaccines: Why and What to Do? Hum. Vaccines Immunother. 2016, 12, 239–243.
- Javelle, E.; Colson, P.; Parola, P.; Raoult, D. Measles, the Need for a Paradigm Shift. Eur. J. Epidemiol. 2019, 34, 897–915.
- Sniadack, D.H.; Crowcroft, N.S.; Durrheim, D.N.; Rota, P.A. Roadmap to Elimination—Standard Measles and Rubella Surveillance. Wkly. Epidemiol. Rec. 2017, 9, 10–92.
- Pannuti, C.S.; Morello, R.J.; de Moraes, J.C.; Curti, S.P.; Afonso, A.M.S.; Camargo, M.C.C.; de Souza, V.A.U.F. Identification of Primary and Secondary Measles Vaccine Failures by Measurement of Immunoglobulin G Avidity in Measles Cases during the 1997 São Paulo Epidemic. Clin. Vaccine Immunol. 2004, 11, 119–122.
- Mercader, S.; Garcia, P.; Bellini, W.J. Measles Virus IgG Avidity Assay for Use in Classification of Measles Vaccine Failure in Measles Elimination Settings. Clin. Vaccine Immunol. 2012, 19, 1810–1817.
- Hubiche, T.; Brazier, C.; Vabret, A.; Reynaud, S.; Roudiere, L.; del Giudice, P. Measles Transmission in a Fully Vaccinated Closed Cohort: Data From a Nosocomial Clustered Cases in a Teenage Psychiatric Unit. Pediatr. Infect. Dis. J. 2019, 38, e230.
- World Health Organization. Manual for the Laboratory-Based Surveillance of Measles, Rubella, and Congenital Rubella Syndrome; World Health Organization: Geneva, Switzerland, 2018.
- Edmonson, M.B.; Addiss, D.G.; McPherson, J.T.; Berg, J.L.; Circo, S.R.; Davis, J.P. Mild Measles and Secondary Vaccine Failure During a Sustained Outbreak in a Highly Vaccinated Population. JAMA 1990, 263, 2467–2471.
- Iwamoto, M.; Hickman, C.J.; Colley, H.; Arciuolo, R.J.; Mahle, C.E.; Deocharan, B.; Siemetzki-Kapoor, U.; Zucker, J.R.; Rosen, J.B. Measles Infection in Persons with Secondary Vaccine Failure, New York City, 2018–2019. Vaccine 2021, 39, 5346–5350.
- Measles and Rubella Strategic Framework: 2021–2030. Available online: https://www.who.int/publications-detail-redirect/measles-and-rubella-strategic-framework-2021-2030 (accessed on 17 May 2022).
- Martha Iwamoto; Carole J. Hickman; Heather Colley; Robert J. Arciuolo; Christine E. Mahle; Bisram Deocharan; Ulrike Siemetzki-Kapoor; Jane R. Zucker; Jennifer B. Rosen; Measles infection in persons with secondary vaccine failure, New York City, 2018–19. Vaccine 2021, 39, 5346-5350, 10.1016/j.vaccine.2021.07.078.
- Augusto, G.F.; Silva, A.; Pereira, N.; Fernandes, T.; Leça, A.; Valente, P.; Calé, E.; Aguiar, B.A.; Martins, A.; Palminha, P.; et al. Report of Simultaneous Measles Outbreaks in Two Different Health Regions in Portugal, February to May 2017: Lessons Learnt and Upcoming Challenges. Eurosurveillance 2019, 24, 1800026.
- Atrasheuskaya, A.V.; Kulak, M.V.; Neverov, A.A.; Rubin, S.; Ignatyev, G.M. Measles Cases in Highly Vaccinated Population of Novosibirsk, Russia, 2000–2005. Vaccine 2008, 26, 2111–2118.
- Dine, M.S.; Hutchins, S.S.; Thomas, A.; Williams, I.; Bellini, W.J.; Redd, S.C. Persistence of Vaccine-Induced Antibody to Measles 26–33 Years after Vaccination. J. Infect. Dis. 2004, 189 (Suppl. S1), S123–S130.
- Holzmann, H.; Hengel, H.; Tenbusch, M.; Doerr, H.W. Eradication of Measles: Remaining Challenges. Med. Microbiol. Immunol. 2016, 205, 201–208.
- Gaythorpe, K.A.; Abbas, K.; Huber, J.; Karachaliou, A.; Thakkar, N.; Woodruff, K.; Li, X.; Echeverria-Londono, S.; VIMC Working Group on COVID-19 Impact on Vaccine Preventable Disease; Ferrari, M.; et al. Impact of COVID-19-Related Disruptions to Measles, Meningococcal A, and Yellow Fever Vaccination in 10 Countries. Elife 2021, 10, e67023.
- Venkatesan, P. Worrying Global Decline in Measles Immunisation. Lancet Microbe 2022, 3, e9.
