You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Laura Pérez-Campos Mayoral.
Ageing is associated with changes in body composition, such as low muscle mass (sarcopenia), decreased grip strength or physical function (dynapenia), and accumulation of fat mass. When the accumulation of fat mass synergistically accompanies low muscle mass or reduced grip strength, it results in sarcopenic obesity and dynapenic obesity, respectively.
- sarcopenic obesity
- ageing
- dynapenic obesity 1. Introduction
1. Sarcopenic Obesity and Dynapenic Obesity
Obesity is a chronic disease [14][1] defined as the abnormal or excessive accumulation of fat [15][2]. It is expressed in various phenotypes, one of which is sarcopenic obesity [1,16][3][4]. The most widely used means of identifying obesity is to calculate the body mass index, taking the weight in kilograms, and dividing by the height in meters squared. Adult obesity is defined as a BMI of ≥30 Kg/m2 [5]. However, due to the endocrine and inflammatory role of adipose tissue, it is also necessary to classify obese conditions based on the distribution and composition of body fat. For this, some phenotypes of obesity have been described: normal weight obese, metabolically obese normal weight, metabolically healthy obese, metabolically unhealthy obese, and sarcopenic obese [1,17][3][6]. In older people, different phenotypes have been reported as: nonobese nondynapenic, overweight nondynapenic, obese nondynapenic, sarcopenic obese, overweight sarcopenic, nonobese dynapenic, and dynapenic obese [18,19,20][7][8][9] (Figure 1).
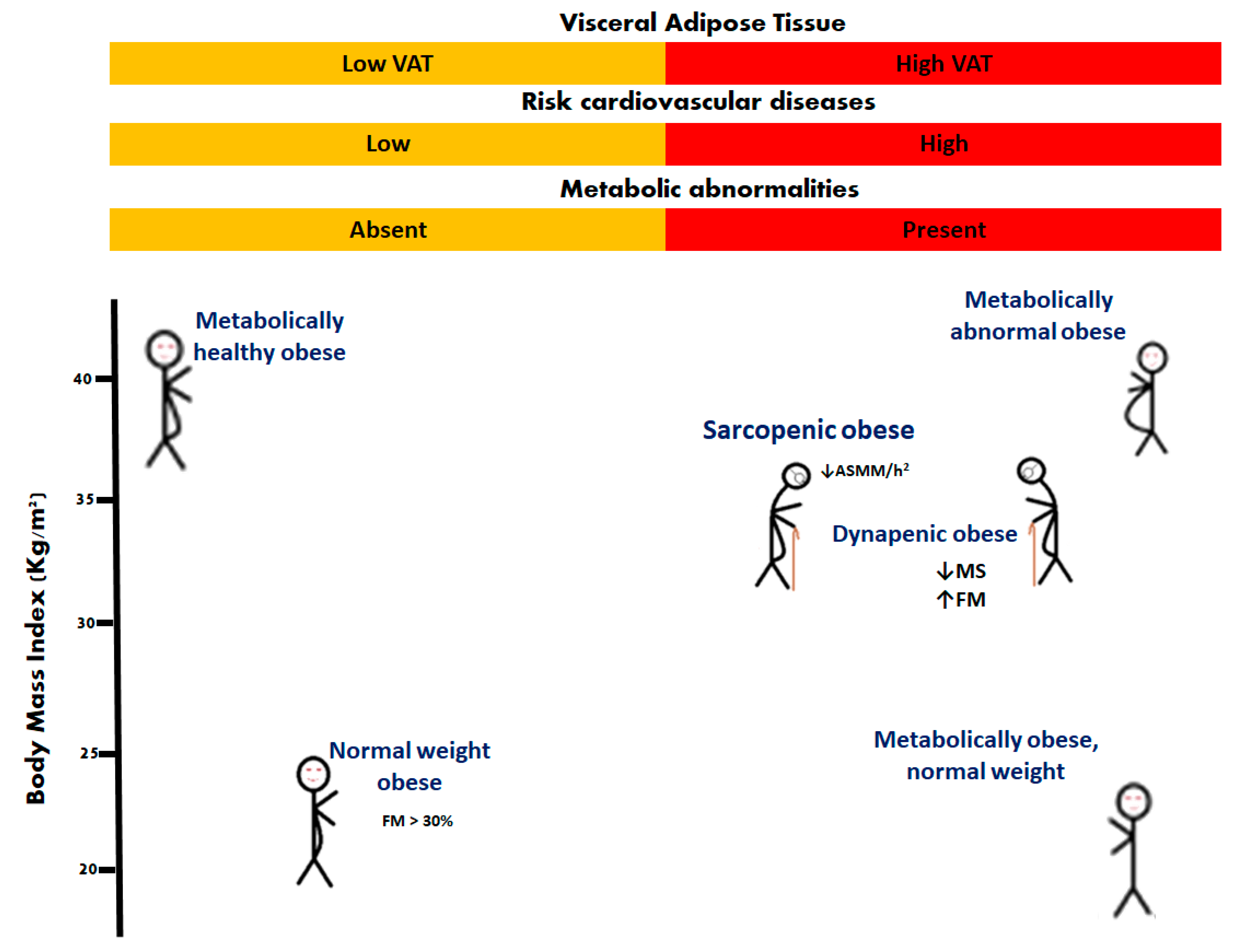
Figure 1. Phenotypes of obesity. In older people, sarcopenic obesity and dynapenic obesity are related to worsening disability. Appendicular skeletal muscle mass divided by height squared (ASMM/h2); fat mass (FM); muscle strength (MS).
It is well known that sarcopenia and obesity disorders are dependent on nutritional status [21][10], body composition, hormonal changes [22][11], strength and muscle mass [23][12], all of which act synergistically to increase the risk of disability [13].
Sarcopenic obesity (SO) is defined as obesity with the loss of muscle mass (ASMM/h2: < 5.18 Kg/m2 in women and <7.00 Kg/m2 in men), while dynapenic obesity (DO) is defined by the association of loss of leg muscle strength (<12 Kg women and <21 Kg men) or physical function; both phenotypes with an accumulation of body fat mass (>40% women and >28% men) or BMI ≥ 30 [24][14], particularly in those with comorbid diseases such as diabetes, arthritis, and cardiovascular and respiratory conditions [25,26,27][15][16][17]. It is important to mention that low muscle mass is associated with dynapenia and decreased motor capacity [28][18]. For the diagnosis of obesity in geriatric patients, the concept of DO is rarely considered; thus, patients with decreased muscle mass or low handgrip strength in sarcopenia may be included [29,30][19][20].
There are several mechanisms and factors related to the pathogenesis of SO and DO (Figure 2), such as (1) adipose tissue dysfunction characterized by adipocyte hyperplasia and hypertrophy [31,32][21][22]; (2) perilipins 5 is related to a decrease in the lipotoxicity and insulin resistance [33][23]; (3) systemic chronic sterile low-grade inflammation [34,35,36][24][25][26]; (4) vitamin D deficiency associated with handgrip strength but not with muscle mass [37][27]; (5) vitamin D receptor gene polymorphism of Fok1 associated with sarcopenia, lower gait speed, and lower handgrip strength [38][28]; (6) adipose tissue inflammation with an accumulation of macrophages and lymphocytes [39,40][29][30]; (7) during inflammation of adipose tissue, the accumulation of M1 macrophages around necrotic adipocytes produces the release of fatty acids. This is associated with the production of a greater amount of tumor necrosis factor-alpha (TNF-α), which releases more fatty acids from adipocytes, becoming a vicious circle that maintains the proinflammatory environment [41][31].
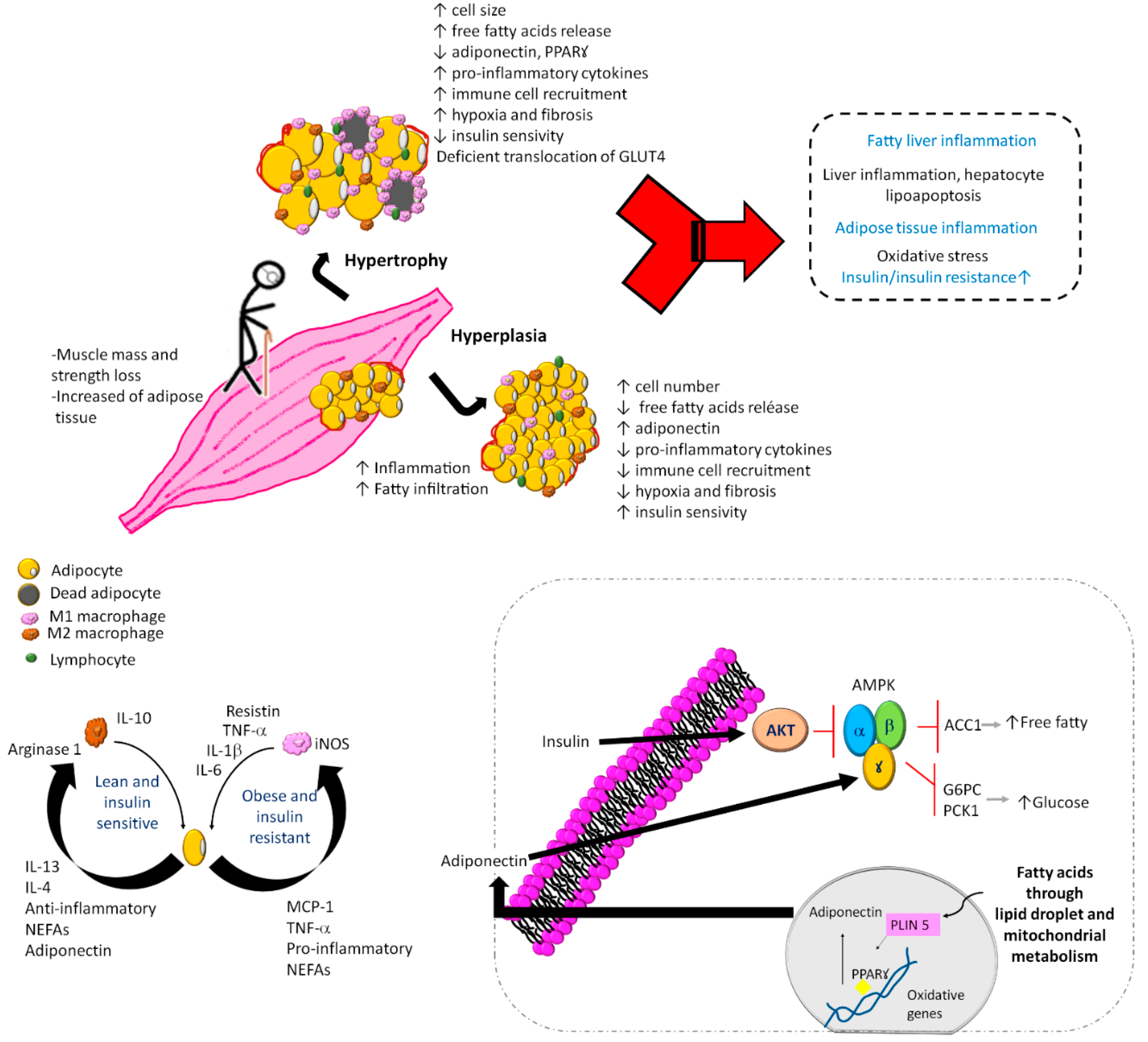
Figure 2. Mechanisms and factors related to the pathogenesis of sarcopenic obesity and dynapenic obesity. In sarcopenic obesity and dynapenic obesity, the muscle mass and strength loss with increased adipose tissue induces an inflammatory cascade and accumulation of immune cells, as well as leukocyte activation, adipogenesis, and adipocyte death. Added to physical inactivity, carbohydrate overload and lower protein intake cause a vicious circle of insulin resistance, where there is an increase in free fatty acids and M1 macrophages with alterations in mitochondrial metabolism by inactivation of regulators of energy homeostasis and inducers of regulated fatty acid oxidation, vitamin D deficiency, and D receptor gene polymorphism. G6PC, glucose-6-phosphatase; PKC1, phosphoenolpyruvate carboxykinase 1; AMPK, AMP-activated protein kinase; ACC1, acetyl-CoA carboxylase 1; NEFAs, nonesterified fatty acids; PLIN 5, perilipin 5; PPARꙋ, peroxisome proliferator-activated receptor gamma; GLUT4, glucose transporter type 4.
All these factors cause an asymptomatic inflammatory condition in hypertrophied adipose tissue with a high number of inflammatory cells, production of adipokines and other inflammatory cytokines [42][32]. The production of inflammatory cytokines motivates the arrival of immune cells, mainly macrophages, interferon gamma-producing TH1 lymphocytes (INF-γ), and CD8+ lymphocytes capable of initiating the inflammatory response [43,44][33][34].
In the skeletal muscle, fat droplets are accumulated as intermuscular adipose tissue and intramyocellular lipids (IMCLs) [45][35]. One characteristic of IMCLs is that they can induce a lipotoxic effect on muscles, which is characterized by impaired single-fiber contractility, leading to lower muscle strength and power in the elderly. This occurs because of the autophagy of muscle cells [46][36]. In the pathogenesis of sarcopenia, an important molecule identified at the neuromuscular junction is the C-terminal agrin fragment, which causes an age-dependent increase and muscle dysfunction [47][37]. Furthermore, during ageing, the change in muscle mass and weight gain (by lean mass) reflect the decrease in metabolic rate [48][38]. In addition, SO is related to elevated levels of IL-6, high-sensitivity C-reactive protein (hs-CRP) [49][39], IL-1 receptor antagonist, and soluble IL-6 receptor; all of these could contribute to apoptosis in myocytes and lead to a decrease in muscle mass and strength [50,51][40][41]. Thus, in SO, frailty and changes in immune function with age (immunosenescence) [52][42] are associated with physical inactivity and the reduction of energy expenditure, as well as impairment of movement and respiratory problems linked to metabolic alterations, leading to increased risk of comorbidity.
Vitamin D deficiency has also been found to be common in obese people [63][43]. This deficiency is associated with an increased risk of frailty, falls, and increased fracture risk in DO and SO [7,64,65][44][45][46]. Nevertheless, there are discrepancies in different disorder studies in which vitamin D is supplemented [36][26]. It should be remembered that vitamin D aids the body to absorb calcium, one of the main nutrients necessary for strong bones; vitamin D levels and vitamin D receptor gene polymorphism should be taken into consideration in SO and DO.
Although the affectations in SO and DO vary from one individual to another, there is a consensus on the higher risk of mortality in older adults in both cases [2][47]. Berens et al. evaluated the possible association between mortality and obesity in people, both with and without sarcopenia, and found that 75-year-old women with SO have a greater risk of dying at 10 years, compared to those without sarcopenia or obesity, while for 87-year-old obese men without sarcopenia, it was associated with a survival limit of up to four years. [66][48].
2. Sarcopenic Obesity, Dynapenic Obesity, and COVID-19
SO could increase the risk of severe complications and adverse outcomes in COVID-19 (Figure 3) [13,101][13][49]. Among the most notable findings of the COVID-19 patient are the effects of myalgia arthralgia, back pain, fatigue, and loss of grip strength [102][50], in addition to the fact that the SARS-CoV-2 infection mainly affects the epithelium of the lungs. Furthermore, other systems have also been involved, such as the immune [103][51], integumentary [104][52], neurological [105][53], digestive [106][54], genitourinary [107][55], cardiovascular [108][56], hematological [109][57], reproductive, and hormonal systems [110[58][59],111], causing very varied symptomatology. In the most severe cases, COVID-19 causes pneumonia, heart and kidney failure, and liver injury, thrombosis, shock, and even death. Twenty-five per cent of these patients develop severe lung disease that progresses to adult respiratory distress syndrome [112,113][60][61].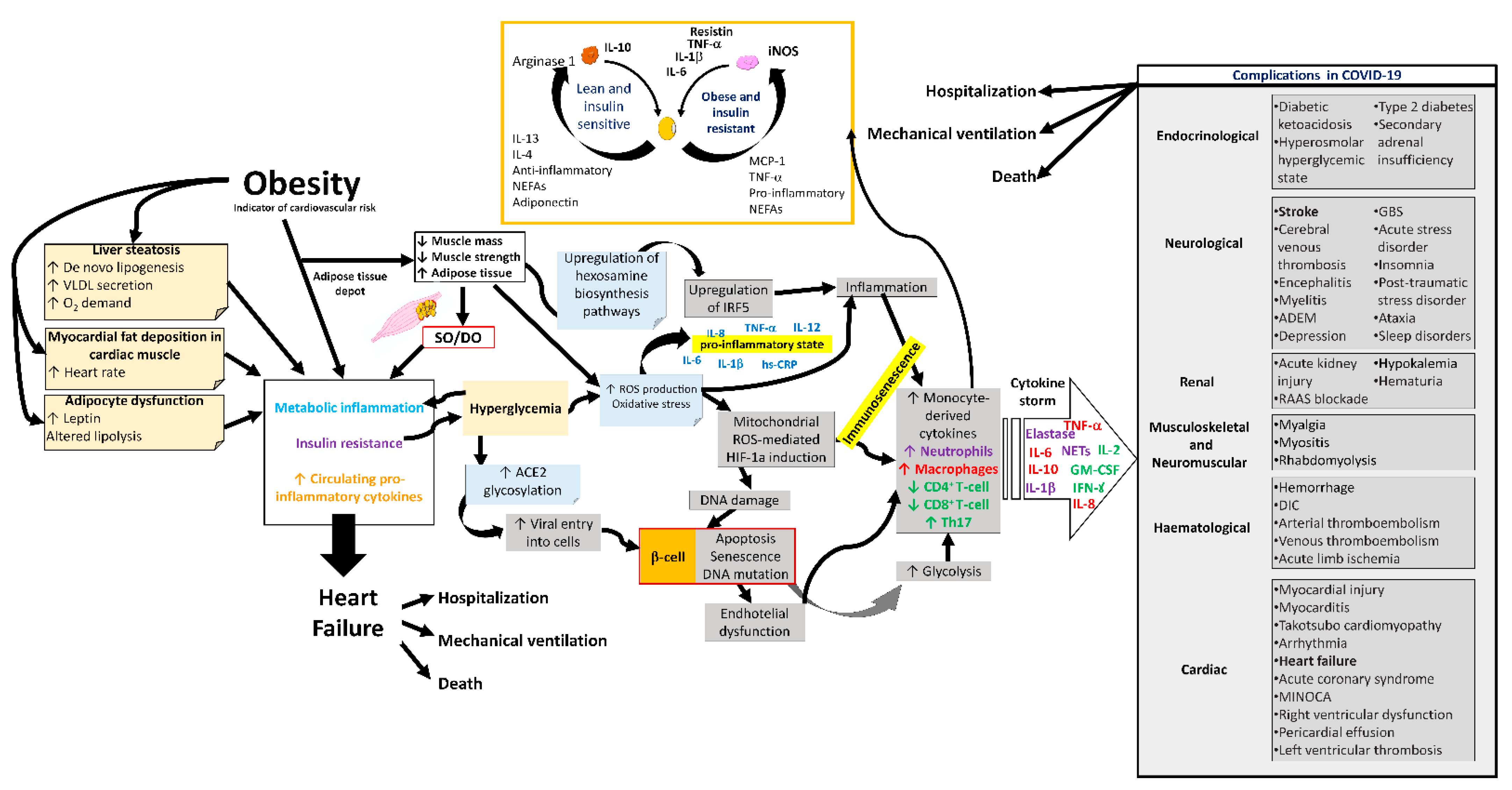
Figure 3. Complications in COVID-19 due to sarcopenic obesity and dynapenic obesity. Alterations in the metabolism, respiratory system, cardiovascular system, and immune system of the SO and/or DO patient with a propensity for complications during COVID-19. SO, sarcopenic obesity; DO, dynapenic obesity; ROS, reactive oxygen species; hs-CRP, high-sensitivity C-reactive protein; ACE2, angiotensin-converting enzyme 2; NETs, neutrophil extracellular traps; GM-CSF, granulocyte macrophage colony-stimulating factor; IFN-γ, gamma interferon; HIF-1a, hypoxia-inducible factor-1α; IRF5, interferon regulatory factor 5; VLDL, very low-density lipoprotein; ADEM, acute disseminated encephalomyelitis; GBS, Guillain–Barré syndrome; RAAS, renin–angiotensin–aldosterone system; DIC, disseminated intravascular coagulation; MINOCA, myocardial infarction with nonobstructive coronaries.
References
- Laviano, A.; Koverech, A.; Zanetti, M. Nutrition support in the time of SARS-CoV-2 (COVID-19). Nutrition 2020, 74, 110834.
- Purnell, J.Q. Definitions, Classification, and Epidemiology of Obesity. . In Endotext ; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK279167/ (accessed on 29 November 2021).
- Mayoral, L.P.; Andrade, G.M.; Mayoral, E.P.; Huerta, T.H.; Canseco, S.P.; Rodal Canales, F.J.; Cabrera-Fuentes, H.A.; Cruz, M.M.; Pérez Santiago, A.D.; Alpuche, J.J.; et al. Obesity subtypes, related biomarkers & heterogeneity. Indian J. Med. Res. 2020, 151, 11–21.
- World Health Organization. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 November 2021).
- Kim, Y.S.; Lee, Y.; Chung, Y.S.; Lee, D.J.; Joo, N.S.; Hong, D.; Song, G.E.; Kim, H.J.; Choi, Y.J.; Kim, K.M. Prevalence of sarcopenia and sarcopenic obesity in the Korean population based on the Fourth Korean National Health and Nutritional Examination Surveys. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1107–1113.
- Lee, D.C.; Shook, R.P.; Drenowatz, C.; Blair, S.N. Physical activity and sarcopenic obesity: Definition, assessment, prevalence and mechanism. Future Sci. OA 2016, 2, FSO127.
- De Lorenzo, A.; Soldati, L.; Sarlo, F.; Calvani, M.; Di Lorenzo, N.; Di Renzo, L. New obesity classification criteria as a tool for bariatric surgery indication. World J. Gastroenterol. 2016, 22, 681–703.
- Xia, M.F.; Chen, L.Y.; Wu, L.; Ma, H.; Li, X.M.; Li, Q.; Aleteng, Q.; Hu, Y.; He, W.Y.; Gao, J.; et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: A cross-sectional study. Clin. Nutr. 2021, 40, 571–580.
- Chain, A.; Faerstein, E.; Wahrlich, V.; Bezerra, F.F. Obesity, dynapenia, and their combination: Implications for bone mineral density in Brazilian adults-the Pró-Saúde study. Nutrition 2021, 81, 110898.
- Wierdsma, N.J.; Kruizenga, H.M.; Konings, L.A.; Krebbers, D.; Jorissen, J.R.; Joosten, M.I.; van Aken, L.H.; Tan, F.M.; van Bodegraven, A.A.; Soeters, M.R.; et al. Poor nutritional status, risk of sarcopenia and nutrition related complaints are prevalent in COVID-19 patients during and after hospital admission. Clin. Nutr. ESPEN 2021, 43, 369–376.
- Gungor, O.; Ulu, S.; Hasbal, N.B.; Anker, S.D.; Kalantar-Zadeh, K. Effects of hormonal changes on sarcopenia in chronic kidney disease: Where are we now and what can we do? J. Cachexia Sarcopenia Muscle 2021, 12, 1380–1392.
- Gil, S.; Jacob, F.W.; Shinjo, S.K.; Ferriolli, E.; Busse, A.L.; Avelino-Silva, T.J.; Longobardi, I.; de Oliveira Júnior, G.N.; Swinton, P.; Gualano, B.; et al. Muscle strength and muscle mass as predictors of hospital length of stay in patients with moderate to severe COVID-19: A prospective observational study. J. Cachexia Sarcopenia Muscle 2021, 12, 1871–1878.
- Kara, M.; Ata, A.M.; Özçakar, L. Sarcopenic obesity is the real problem in COVID-19! Eur. J. Intern. Med. 2021, 93, 103–104.
- Rossi, A.P.; Urbani, S.; Fantin, F.; Nori, N.; Brandimarte, P.; Martini, A.; Zoico, E.; Mazzali, G.; Babbanini, A.; Muollo, V.; et al. Worsening Disability and Hospitalization Risk in Sarcopenic Obese and Dynapenic Abdominal Obese: A 5.5 Years Follow-Up Study in Elderly Men and Women. Front. Endocrinol. 2020, 11, 314.
- Waters, D.L.; Baumgartner, R.N. Sarcopenia and obesity. Clin. Geriatr. Med. 2011, 27, 401–421.
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537.
- Bouchard, D.R.; Janssen, I. Dynapenic-obesity and physical function in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 71–77.
- Sattar, N.; McInnes, I.B.; McMurray, J. Obesity Is a Risk Factor for Severe COVID-19 Infection: Multiple Potential Mechanisms. Circulation 2020, 142, 4–6.
- Hong, S.H.; Choi, K.M. Sarcopenic Obesity, Insulin Resistance, and Their Implications in Cardiovascular and Metabolic Consequences. Int. J. Mol. Sci. 2020, 21, 494.
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity: ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335.
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30.
- Divella, R.; De Luca, R.; Abbate, I.; Naglieri, E.; Daniele, A. Obesity and cancer: The role of adipose tissue and adipo-cytokines-induced chronic inflammation. J. Cancer 2016, 7, 2346–2359.
- Jarc, E.; Petan, T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019, 92, 435–452.
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558.
- Zamboni, M.; Mazzali, G.; Fantin, F.; Rossi, A.; Di Francesco, V. Sarcopenic obesity: A new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 388–395.
- Sabico, S.; Al-Daghri, N.M. Sarcopenic Obesity. In Sarcopenia. Practical Issues in Geriatrics; Veronese, N., Beaudart, C., Sabico, S., Eds.; Springer: Cham, Switzerland, 2021; Volume 145–151.
- Gumieiro, D.N.; Murino, R.B.; Buzati, P.B.; Cavallari, K.A.; Tanni, S.E.; Azevedo, P.S.; Polegato, B.F.; Mamede Zornoff, L.A.; Dinhane, D.I.; Innocenti Dinhane, K.G.; et al. Vitamin D serum levels are associated with handgrip strength but not with muscle mass or length of hospital stay after hip fracture. Nutrition 2015, 31, 931–934.
- Yao, X.; Yang, L.; Li, M.; Xiao, H. Relationship of vitamin D receptor gene polymorphism with sarcopenia and muscle traits based on propensity score matching. J. Clin. Lab. Anal. 2020, 34, e23485.
- Rogero, M.M.; Calder, P.C. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432.
- Weng, S.Y.; Schuppan, D. AMPK regulates macrophage polarization in adipose tissue inflammation and NASH. J. Hepatol. 2013, 58, 619–621.
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221.
- Engin, A.B. Adipocyte-Macrophage Cross-Talk in Obesity. Adv. Exp. Med. Biol. 2017, 960, 327–343.
- Thomas, D.; Apovian, C. Macrophage functions in lean and obese adipose tissue. Metabolism 2017, 72, 120–143.
- Asghar, A.; Sheikh, N. Role of immune cells in obesity induced low grade inflammation and insulin resistance. Cell Immunol. 2017, 315, 18–26.
- Schrauwen-Hinderling, V.B.; Hesselink, M.K.; Schrauwen, P.; Kooi, M.E. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring, Md.) 2006, 14, 357–367.
- Agrawal, M.; Kern, P.A.; Nikolajczyk, B.S. The Immune System in Obesity: Developing Paradigms Amidst Inconvenient Truths. Curr. Diab. Rep. 2017, 17, 87.
- Kalinkovich, A.; Livshits, G. Sarcopenia—The search for emerging biomarkers. Ageing Res. Rev. 2015, 22, 58–71.
- Weinsier, R.L.; Schutz, Y.; Bracco, D. Reexamination of the relationship of resting metabolic rate to fat-free mass and to the metabolically active components of fat-free mass in humans. Am. J. Clin. Nutr. 1992, 55, 790–794.
- Yang, C.W.; Li, C.I.; Li, T.C.; Liu, C.S.; Lin, C.H.; Lin, W.Y.; Lin, C.C. Association of Sarcopenic Obesity with Higher Serum High-Sensitivity C-Reactive Protein Levels in Chinese Older Males—A Community-Based Study (Taichung Community Health Study-Elderly, TCHS-E). PLoS ONE 2015, 10, e0132908.
- Schrager, M.A.; Metter, E.J.; Simonsick, E.; Ble, A.; Bandinelli, S.; Lauretani, F.; Ferrucci, L. Sarcopenic obesity and inflammation in the InCHIANTI study. J. Appl. Physiol. 2007, 102, 919–925.
- Aubertin-Leheudre, M.; Anton, S.; Beavers, D.P.; Manini, T.M.; Fielding, R.; Newman, A.; Church, T.; Kritchevsky, S.B.; Conroy, D.; McDermott, M.M.; et al. Dynapenia and Metabolic Health in Obese and Nonobese Adults Aged 70 Years and Older: The LIFE Study. J. Am. Med. Dir. Assoc. 2017, 18, 312–319.
- Lian, J.; Yue, Y.; Yu, W.; Zhang, Y. Immunosenescence: A key player in cancer development. J. Hematol. Oncol. 2020, 13, 151.
- Walsh, J.S.; Evans, A.L.; Bowles, S.; Naylor, K.E.; Jones, K.S.; Schoenmakers, I.; Jacques, R.M.; Eastell, R. Free 25-hydroxyvitamin D is low in obesity, but there are no adverse associations with bone health. Am. J. Clin. Nutr. 2016, 103, 1465–1471.
- Scott, D.; Sanders, K.M.; Aitken, D.; Hayes, A.; Ebeling, P.R.; Jones, G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity 2014, 22, 1568–1574.
- Millward, D.J. Nutrition and sarcopenia: Evidence for an interaction. Proc. Nutr. Soc. 2012, 71, 566–575.
- Lima, R.M.; de Oliveira, R.J.; Raposo, R.; Neri, S.; Gadelha, A.B. Stages of sarcopenia, bone mineral density, and the prevalence of osteoporosis in older women. Arch. Osteoporos. 2019, 14, 38.
- Petermann-Rocha, F.; Yang, S.; Gray, S.R.; Pell, J.P.; Celis-Morales, C.; Ho, F.K. Sarcopenic obesity and its association with respiratory disease incidence and mortality. Clin. Nutr. 2020, 39, 3461–3466.
- von Berens, Å.; Obling, S.R.; Nydahl, M.; Koochek, A.; Lissner, L.; Skoog, I.; Frändin, K.; Skoglund, E.; Rothenberg, E.; Cederholm, T. Sarcopenic obesity and associations with mortality in older women and men—A prospective observational study. BMC Geriatr. 2020, 20, 199.
- Damanti, S.; Cristel, G.; Ramirez, G.A.; Bozzolo, E.P.; Da Prat, V.; Gobbi, A.; Centurioni, C.; Di Gaeta, E.; Del Prete, A.; Calabrò, M.G.; et al. Influence of reduced muscle mass and quality on ventilator weaning and complications during intensive care unit stay in COVID-19 patients. Clin. Nutr. 2021, S0261–5614, 00375–00377.
- Tuzun, S.; Keles, A.; Okutan, D.; Yildiran, T.; Palamar, D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur. J. Phys. Rehabil. Med. 2021, 57, 653–662.
- Li, G.; Fan, Y.; Lai, Y.; Han, T.; Li, Z.; Zhou, P.; Pan, P.; Wang, W.; Hu, D.; Liu, X.; et al. Coronavirus infections and immune responses. J. Med. Virol. 2020, 92, 424–432.
- Wollina, U.; Karadağ, A.S.; Rowland-Payne, C.; Chiriac, A.; Lotti, T. Cutaneous signs in COVID-19 patients: A review. Dermatol. Ther. 2020, 33, e13549.
- Baig, A.M.; Khaleeq, A.; Ali, U.; Syeda, H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem. Neurosci. 2020, 11, 995–998.
- Matthai, J.; Shanmugam, N.; Sobhan, P.; Indian Society of Pediatric Gastroenterology, Hepatology and Nutrition; Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics. Coronavirus Disease (COVID-19) and the Gastrointestinal System in Children. Indian Pediatr. 2020, 57, 533–535.
- Su, H.; Yang, M.; Wan, C.; Yi, L.X.; Tang, F.; Zhu, H.Y.; Yi, F.; Yang, H.C.; Fogo, A.B.; Nie, X.; et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227.
- Zhu, H.; Rhee, J.W.; Cheng, P.; Waliany, S.; Chang, A.; Witteles, R.M.; Maecker, H.; Davis, M.M.; Nguyen, P.K.; Wu, S.M. Cardiovascular Complications in Patients with COVID-19: Consequences of Viral Toxicities and Host Immune Response. Curr. Cardiol. Rep. 2020, 22, 36.
- Liu, X.; Zhang, R.; He, G. Hematological findings in coronavirus disease 2019: Indications of progression of disease. Ann. Hematol. 2020, 99, 1421–1428.
- La Vignera, S.; Cannarella, R.; Condorelli, R.A.; Torre, F.; Aversa, A.; Calogero, A.E. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int. J. Mol. Sci. 2020, 21, 2948.
- Jing, Y.; Run-Qian, L.; Hao-Ran, W.; Hao-Ran, C.; Ya-Bin, L.; Yang, G.; Fei, C. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol. Hum. Reprod. 2020, 26, 367–373.
- See, A.; Toh, S.T. Respiratory sampling for severe acute respiratory syndrome coronavirus 2: An Overview. Head Neck 2020, 42, 1652–1656.
- Elsevier Connect. El Nuevo Coronavirus SARS-CoV-2 y su Enfermedad, COVID-19, ¿a qué nos Enfrentamos? Available online: https://www.elsevier.com/es-es/connect/coronavirus/SARS-CoV-2-y-su-enfermedad-COVID19-a-que-nos-enfrentamos (accessed on 15 December 2021).
- Ekiz, T.; Pazarl, A.C. Relationship between COVID-19 and obesity. Diabetes Metab. Syndr. 2020, 14, 761–763.
- Li, M.Y.; Li, L.; Zhang, Y.; Wang, X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect. Dis. Poverty 2020, 9, 45.
- Fang, L.; Karakiulakis, G.; Roth, M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir. Med. 2020, 8, e21.
- Kreidieh, D.; Itani, L.; El Masri, D.; Tannir, H.; Citarella, R.; El Ghoch, M. Association between Sarcopenic Obesity, Type 2 Diabetes, and Hypertension in Overweight and Obese Treatment-Seeking Adult Women. J. Cardiovasc. Dev. Dis. 2018, 5, 51.
- Liu, K.; Chen, Y.; Lin, R.; Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 2020, 80, e14–e18.
- Frühbeck, G.; Baker, J.L.; Busetto, L.; Dicker, D.; Goossens, G.; Halford, J.; Handjieva-Darlenska, T.; Hassapidou, M.; Holm, J.C.; Lehtinen-Jacks, S.; et al. European Association for the Study of Obesity Position Statement on the Global COVID-19 Pandemic. Obes. Facts 2020, 13, 292–296.
- Gupta, R.; Ghosh, A.; Singh, A.K.; Misra, A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab. Syndr. 2020, 14, 211–212.
- Molfino, A.; Imbimbo, G.; Rizzo, V.; Muscaritoli, M.; Alampi, D. The link between nutritional status and outcomes in COVID-19 patients in ICU: Is obesity or sarcopenia the real problem? Eur. J. Intern. Med. 2021, 91, 93–95.
- Besutti, G.; Pellegrini, M.; Ottone, M.; Cantini, M.; Milic, J.; Bonelli, E.; Dolci, G.; Cassone, G.; Ligabue, G.; Spaggiari, L.; et al. The impact of chest CT body composition parameters on clinical outcomes in COVID-19 patients. PLoS ONE 2021, 16, e0251768.
- Rahmati-Ahmadabad, S.; Hosseini, F. Exercise against SARS-CoV-2 (COVID-19): Does workout intensity matter? (A mini review of some indirect evidence related to obesity). Obes. Med. 2020, 19, 100245.
- Reiterer, M.; Rajan, M.; Gómez-Banoy, N.; Lau, J.D.; Gomez-Escobar, L.G.; Ma, L.; Gilani, A.; Alvarez-Mulett, S.; Sholle, E.T.; Chandar, V.; et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. 2021, 33, 2174–2188.e5.
- Wang, A.; Zhao, W.; Xu, Z.; Gu, J. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res. Clin. Pract. 2020, 162, 108118.
- Roh, E.; Choi, K.M. Health Consequences of Sarcopenic Obesity: A Narrative Review. Front. Endocrinol. 2020, 11, 332.
- Wilkinson, T.J.; Yates, T.; Baker, L.A.; Zaccardi, F.; Smith, A.C. Sarcopenic obesity and the risk of hospitalization or death from coronavirus disease 2019: Findings from UK Biobank. JCSM Rapid Commun. 2021, 5, 3–9.
- Romero-Cabrera, Á.J.; Amores-Hernández, L.; Fernández- Casteleiro, E. Immunosenescence and Frailty: A Current Glance. Med. Int. Mex. 2013, 29, 605–611.
- Nelke, C.; Dziewas, R.; Minnerup, J.; Meuth, S.G.; Ruck, T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine 2019, 49, 381–388.
- Kara, Ö.; Kara, M.; Akın, M.E.; Özçakar, L. Grip strength as a predictor of disease severity in hospitalized COVID-19 patients. Heart Lung 2021, 50, 743–747.
More
