Acute Myeloid Leukemia (AML) is an aggressive hematological malignancy that relies on highly heterogeneous cytogenetic alterations. Although in the last few years new agents have been developed for AML treatment, the overall survival prospects for AML patients are still gloomy and new therapeutic options are still urgently needed. Constitutive NF-κB activation has been reported in around 40% of AML patients, where it sustains AML cell survival and chemoresistance. Given the central role of NF-κB in AML, targeting the NF-κB pathway represents an attractive strategy to treat AML. The focuses are on current knowledge of NF-κB’s roles in AML pathogenesis and summarizes the main therapeutic approaches used to treat NF-κB-driven AML.
- NF-κB
- acute myeloid leukemia
- NF-κB inhibitors
1. NF-κB Pathway in Acute Myeloid Leukemia (AML) Pathogenesis
2. Genetic Alterations Drive NF-κB Constitutive Activation in AML
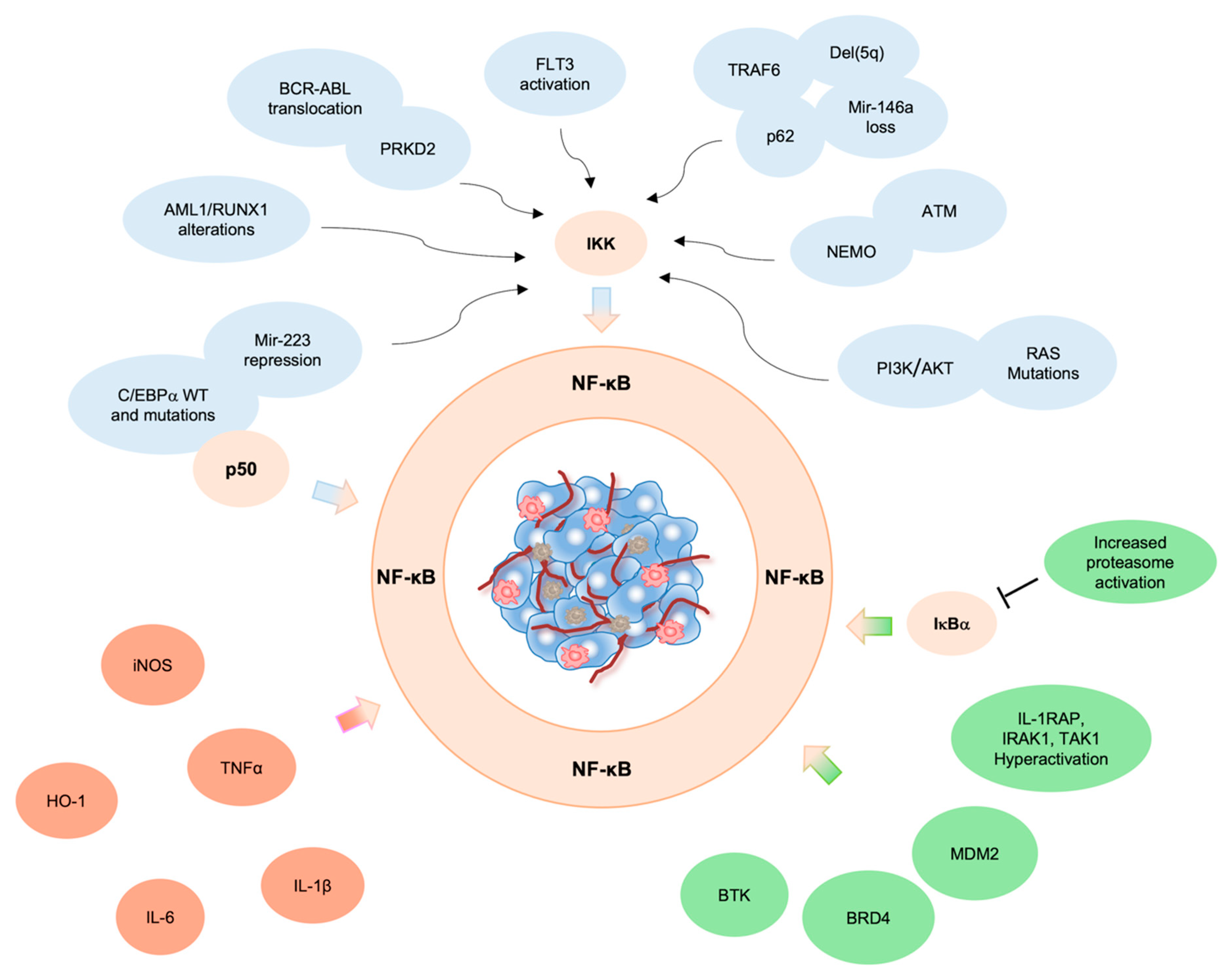
2.1. ATM
2.2. BCR/ABL
2.3. RUNX1
2.4. C/EBPα
2.5. q Deletion
2.6. RAS
3. Pro-Inflammatory Microenvironment and NF-κB Activation in Leukemic Cells
4. Aberrant NF-κB Activation by NF-κB Regulators/Interactors
References
- Labbozzetta, M.; Notarbartolo, M.; Poma, P. Can NF-ΚB Be Considered a Valid Drug Target in Neoplastic Diseases? Our Point of View. Int. J. Mol. Sci. 2020, 21, 3070.
- Bosman, M.C.J.; Schuringa, J.J.; Vellenga, E. Constitutive NF-ΚB Activation in AML: Causes and Treatment Strategies. Crit. Rev. Oncol./Hematol. 2016, 98, 35–44.
- Gasparini, C.; Celeghini, C.; Monasta, L.; Zauli, G. NF-ΚB Pathways in Hematological Malignancies. Cell. Mol. Life Sci. CMLS 2014, 71, 2083–2102.
- Guzman, M.L.; Neering, S.J.; Upchurch, D.; Grimes, B.; Howard, D.S.; Rizzieri, D.A.; Luger, S.M.; Jordan, C.T. Nuclear Factor-KappaB Is Constitutively Activated in Primitive Human Acute Myelogenous Leukemia Cells. Blood 2001, 98, 2301–2307.
- Baumgartner, B.; Weber, M.; Quirling, M.; Fischer, C.; Page, S.; Adam, M.; Von Schilling, C.; Waterhouse, C.; Schmid, C.; Neumeier, D.; et al. Increased IkappaB Kinase Activity Is Associated with Activated NF-KappaB in Acute Myeloid Blasts. Leukemia 2002, 16, 2062–2071.
- Mehta, S.V.; Shukla, S.N.; Vora, H.H. Overexpression of Bcl2 Protein Predicts Chemoresistance in Acute Myeloid Leukemia: Its Correlation with FLT3. Neoplasma 2013, 60, 666–675.
- Wei, T.-Y.W.; Wu, P.-Y.; Wu, T.-J.; Hou, H.-A.; Chou, W.-C.; Teng, C.-L.J.; Lin, C.-R.; Chen, J.-M.M.; Lin, T.-Y.; Su, H.-C.; et al. Aurora a and NF-ΚB Survival Pathway Drive Chemoresistance in Acute Myeloid Leukemia via the TRAF-Interacting Protein TIFA. Cancer Res. 2017, 77, 494–508.
- Notarbartolo, M.; Cervello, M.; Dusonchet, L.; Cusimano, A.; D’Alessandro, N. Resistance to Diverse Apoptotic Triggers in Multidrug Resistant HL60 Cells and Its Possible Relationship to the Expression of P-Glycoprotein, Fas and of the Novel Anti-Apoptosis Factors IAP (Inhibitory of Apoptosis Proteins). Cancer Lett. 2002, 180, 91–101.
- Notarbartolo, M.; Cervello, M.; Giannitrapani, L.; Meli, M.; Poma, P.; Dusonchet, L.; Montalto, G.; D’Alessandro, N. Expression of IAPs and Alternative Splice Variants in Hepatocellular Carcinoma Tissues and Cells. Ann. N. Y. Acad. Sci. 2004, 1028, 289–293.
- Tamm, I.; Kornblau, S.M.; Segall, H.; Krajewski, S.; Welsh, K.; Kitada, S.; Scudiero, D.A.; Tudor, G.; Qui, Y.H.; Monks, A.; et al. Expression and Prognostic Significance of IAP-Family Genes in Human Cancers and Myeloid Leukemias. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 1796–1803.
- Hrdinka, M.; Yabal, M. Inhibitor of Apoptosis Proteins in Human Health and Disease. Genes Immun. 2019, 20, 641–650.
- Marchand, T.; Pinho, S. Leukemic Stem Cells: From Leukemic Niche Biology to Treatment Opportunities. Front. Immunol. 2021, 12, 775128.
- Zhou, J.; Chooi, J.Y.; Ching, Y.Q.; Quah, J.Y.; Toh, S.H.M.; Ng, Y.; Tan, T.Z.; Chng, W.J. NF-ΚB Promotes the Stem-like Properties of Leukemia Cells by Activation of LIN28B. World J. Stem Cells 2018, 10, 34–42.
- Grosjean-Raillard, J.; Tailler, M.; Adès, L.; Perfettini, J.-L.; Fabre, C.; Braun, T.; De Botton, S.; Fenaux, P.; Kroemer, G. ATM Mediates Constitutive NF-KappaB Activation in High-Risk Myelodysplastic Syndrome and Acute Myeloid Leukemia. Oncogene 2009, 28, 1099–1109.
- Shiloh, Y.; Ziv, Y. The ATM Protein Kinase: Regulating the Cellular Response to Genotoxic Stress, and More. Nat. Rev. Mol. Cell Biol. 2013, 14, 197–210.
- Grosjean-Raillard, J.; Adès, L.; Boehrer, S.; Tailler, M.; Fabre, C.; Braun, T.; De Botton, S.; Israel, A.; Fenaux, P.; Kroemer, G. Flt3 Receptor Inhibition Reduces Constitutive NFkappaB Activation in High-Risk Myelodysplastic Syndrome and Acute Myeloid Leukemia. Apoptosis Int. J. Program. Cell Death 2008, 13, 1148–1161.
- Neuendorff, N.R.; Burmeister, T.; Dörken, B.; Westermann, J. BCR-ABL-Positive Acute Myeloid Leukemia: A New Entity? Analysis of Clinical and Molecular Features. Ann. Hematol. 2016, 95, 1211–1221.
- Neuendorff, N.R.; Hemmati, P.; Arnold, R.; Ihlow, J.; Dörken, B.; Müller-Tidow, C.; Westermann, J. BCR-ABL(+) Acute Myeloid Leukemia: Are We Always Dealing with a High-Risk Disease? Blood Adv. 2018, 2, 1409–1411.
- Mariotti, B.; Meconi, F.; Palmieri, R.; De Bellis, E.; Lavorgna, S.; Ottone, T.; Martini, V.; Lo-Coco, F.; Cicconi, L. Acute Myeloid Leukemia with Concomitant BCR-ABL and NPM1 Mutations. Case Rep. Hematol. 2019, 2019, 6707506.
- Hsieh, M.-Y.; Van Etten, R.A. IKK-Dependent Activation of NF-ΚB Contributes to Myeloid and Lymphoid Leukemogenesis by BCR-ABL1. Blood 2014, 123, 2401–2411.
- Mihailovic, T.; Marx, M.; Auer, A.; Van Lint, J.; Schmid, M.; Weber, C.; Seufferlein, T. Protein Kinase D2 Mediates Activation of Nuclear Factor KappaB by Bcr-Abl in Bcr-Abl+ Human Myeloid Leukemia Cells. Cancer Res. 2004, 64, 8939–8944.
- Schnittger, S.; Dicker, F.; Kern, W.; Wendland, N.; Sundermann, J.; Alpermann, T.; Haferlach, C.; Haferlach, T. RUNX1 Mutations Are Frequent in de Novo AML with Noncomplex Karyotype and Confer an Unfavorable Prognosis. Blood 2011, 117, 2348–2357.
- Miyoshi, H.; Shimizu, K.; Kozu, T.; Maseki, N.; Kaneko, Y.; Ohki, M. T(8;21) Breakpoints on Chromosome 21 in Acute Myeloid Leukemia Are Clustered within a Limited Region of a Single Gene, AML1. Proc. Natl. Acad. Sci. USA 1991, 88, 10431–10434.
- Marcucci, G.; Caligiuri, M.A.; Bloomfield, C.D. Molecular and Clinical Advances in Core Binding Factor Primary Acute Myeloid Leukemia: A Paradigm for Translational Research in Malignant Hematology. Cancer Investig. 2000, 18, 768–780.
- Fazi, F.; Racanicchi, S.; Zardo, G.; Starnes, L.M.; Mancini, M.; Travaglini, L.; Diverio, D.; Ammatuna, E.; Cimino, G.; Lo-Coco, F.; et al. Epigenetic Silencing of the Myelopoiesis Regulator MicroRNA-223 by the AML1/ETO Oncoprotein. Cancer Cell 2007, 12, 457–466.
- Fukao, T.; Fukuda, Y.; Kiga, K.; Sharif, J.; Hino, K.; Enomoto, Y.; Kawamura, A.; Nakamura, K.; Takeuchi, T.; Tanabe, M. An Evolutionarily Conserved Mechanism for MicroRNA-223 Expression Revealed by MicroRNA Gene Profiling. Cell 2007, 129, 617–631.
- Li, T.; Morgan, M.J.; Choksi, S.; Zhang, Y.; Kim, Y.-S.; Liu, Z. MicroRNAs Modulate the Noncanonical Transcription Factor NF-KappaB Pathway by Regulating Expression of the Kinase IKKalpha during Macrophage Differentiation. Nat. Immunol. 2010, 11, 799–805.
- Nakagawa, M.; Shimabe, M.; Watanabe-Okochi, N.; Arai, S.; Yoshimi, A.; Shinohara, A.; Nishimoto, N.; Kataoka, K.; Sato, T.; Kumano, K.; et al. AML1/RUNX1 Functions as a Cytoplasmic Attenuator of NF-ΚB Signaling in the Repression of Myeloid Tumors. Blood 2011, 118, 6626–6637.
- Paz-Priel, I.; Friedman, A. C/EBPα Dysregulation in AML and ALL. Crit. Rev. Oncog. 2011, 16, 93–102.
- Roe, J.-S.; Vakoc, C.R. C/EBPα: Critical at the Origin of Leukemic Transformation. J. Exp. Med. 2014, 211, 1–4.
- Grardel, N.; Roumier, C.; Soenen, V.; Lai, J.L.; Plantier, I.; Gheveart, C.; Cosson, A.; Fenaux, P.; Preudhomme, C. Acute Myeloblastic Leukemia (AML) with Inv (16)(P13;Q22) and the Rare I Type CBFbeta-MYH11 Transcript: Report of Two New Cases. Leukemia 2002, 16, 150–151.
- Green, C.L.; Koo, K.K.; Hills, R.K.; Burnett, A.K.; Linch, D.C.; Gale, R.E. Prognostic Significance of CEBPA Mutations in a Large Cohort of Younger Adult Patients with Acute Myeloid Leukemia: Impact of Double CEBPA Mutations and the Interaction with FLT3 and NPM1 Mutations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 2739–2747.
- Paz-Priel, I.; Cai, D.H.; Wang, D.; Kowalski, J.; Blackford, A.; Liu, H.; Heckman, C.A.; Gombart, A.F.; Koeffler, H.P.; Boxer, L.M.; et al. CCAAT/Enhancer Binding Protein Alpha (C/EBPalpha) and C/EBPalpha Myeloid Oncoproteins Induce Bcl-2 via Interaction of Their Basic Regions with Nuclear Factor-KappaB P50. Mol. Cancer Res. MCR 2005, 3, 585–596.
- Paz-Priel, I.; Ghosal, A.K.; Kowalski, J.; Friedman, A.D. C/EBPalpha or C/EBPalpha Oncoproteins Regulate the Intrinsic and Extrinsic Apoptotic Pathways by Direct Interaction with NF-KappaB P50 Bound to the Bcl-2 and FLIP Gene Promoters. Leukemia 2009, 23, 365–374.
- Pulikkan, J.A.; Peramangalam, P.S.; Dengler, V.; Ho, P.A.; Preudhomme, C.; Meshinchi, S.; Christopeit, M.; Nibourel, O.; Müller-Tidow, C.; Bohlander, S.K.; et al. C/EBPα Regulated MicroRNA-34a Targets E2F3 during Granulopoiesis and Is down-Regulated in AML with CEBPA Mutations. Blood 2010, 116, 5638–5649.
- Ebert, B.L. Deletion 5q in Myelodysplastic Syndrome: A Paradigm for the Study of Hemizygous Deletions in Cancer. Leukemia 2009, 23, 1252–1256.
- Fang, J.; Barker, B.; Bolanos, L.; Liu, X.; Jerez, A.; Makishima, H.; Christie, S.; Chen, X.; Rao, D.S.; Grimes, H.L.; et al. Myeloid Malignancies with Chromosome 5q Deletions Acquire a Dependency on an Intrachromosomal NF-ΚB Gene Network. Cell Rep. 2014, 8, 1328–1338.
- Taganov, K.D.; Boldin, M.P.; Chang, K.-J.; Baltimore, D. NF-KappaB-Dependent Induction of MicroRNA MiR-146, an Inhibitor Targeted to Signaling Proteins of Innate Immune Responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486.
- Boldin, M.P.; Taganov, K.D.; Rao, D.S.; Yang, L.; Zhao, J.L.; Kalwani, M.; Garcia-Flores, Y.; Luong, M.; Devrekanli, A.; Xu, J.; et al. MiR-146a Is a Significant Brake on Autoimmunity, Myeloproliferation, and Cancer in Mice. J. Exp. Med. 2011, 208, 1189–1201.
- Zhao, J.L.; Rao, D.S.; Boldin, M.P.; Taganov, K.D.; O’Connell, R.M.; Baltimore, D. NF-KappaB Dysregulation in MicroRNA-146a-Deficient Mice Drives the Development of Myeloid Malignancies. Proc. Natl. Acad. Sci. USA 2011, 108, 9184–9189.
- Beaupre, D.M.; Kurzrock, R. RAS and Leukemia: From Basic Mechanisms to Gene-Directed Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1999, 17, 1071–1079.
- Reuter, C.W.; Morgan, M.A.; Bergmann, L. Targeting the Ras Signaling Pathway: A Rational, Mechanism-Based Treatment for Hematologic Malignancies? Blood 2000, 96, 1655–1669.
- Ward, A.F.; Braun, B.S.; Shannon, K.M. Targeting Oncogenic Ras Signaling in Hematologic Malignancies. Blood 2012, 120, 3397–3406.
- Birkenkamp, K.U.; Geugien, M.; Schepers, H.; Westra, J.; Lemmink, H.H.; Vellenga, E. Constitutive NF-KappaB DNA-Binding Activity in AML Is Frequently Mediated by a Ras/PI3-K/PKB-Dependent Pathway. Leukemia 2004, 18, 103–112.
- Takahashi, S.; Harigae, H.; Ishii, K.K.; Inomata, M.; Fujiwara, T.; Yokoyama, H.; Ishizawa, K.; Kameoka, J.; Licht, J.D.; Sasaki, T.; et al. Over-Expression of Flt3 Induces NF-KappaB Pathway and Increases the Expression of IL-6. Leuk. Res. 2005, 29, 893–899.
- Shanmugam, R.; Gade, P.; Wilson-Weekes, A.; Sayar, H.; Suvannasankha, A.; Goswami, C.; Li, L.; Gupta, S.; Cardoso, A.A.; Baghdadi, T.A.; et al. A Noncanonical Flt3ITD/NF-ΚB Signaling Pathway Represses DAPK1 in Acute Myeloid Leukemia. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 360–369.
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41.
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899.
- Terzić, J.; Grivennikov, S.; Karin, E.; Karin, M. Inflammation and Colon Cancer. Gastroenterology 2010, 138, 2101–2114.e5.
- Rushworth, S.A.; MacEwan, D.J. HO-1 Underlies Resistance of AML Cells to TNF-Induced Apoptosis. Blood 2008, 111, 3793–3801.
- Heasman, S.-A.; Zaitseva, L.; Bowles, K.M.; Rushworth, S.A.; Macewan, D.J. Protection of Acute Myeloid Leukaemia Cells from Apoptosis Induced by Front-Line Chemotherapeutics Is Mediated by Haem Oxygenase-1. Oncotarget 2011, 2, 658–668.
- Olson, S.Y.; Garbán, H.J. Regulation of Apoptosis-Related Genes by Nitric Oxide in Cancer. Nitric Oxide Biol. Chem. 2008, 19, 170–176.
- Brandão, M.M.; Soares, E.; Salles, T.S.; Saad, S.T. Expression of Inducible Nitric Oxide Synthase Is Increased in Acute Myeloid Leukaemia. Acta Haematol. 2001, 106, 95–99.
- Capece, D.; D’Andrea, D.; Verzella, D.; Tornatore, L.; Begalli, F.; Bennett, J.; Zazzeroni, F.; Franzoso, G. Turning an Old GADDget into a Troublemaker. Cell Death Differ. 2018, 25, 642–644.
- Zhang, B.; Ho, Y.W.; Huang, Q.; Maeda, T.; Lin, A.; Lee, S.-U.; Hair, A.; Holyoake, T.L.; Huettner, C.; Bhatia, R. Altered Microenvironmental Regulation of Leukemic and Normal Stem Cells in Chronic Myelogenous Leukemia. Cancer Cell 2012, 21, 577–592.
- Jacamo, R.; Chen, Y.; Wang, Z.; Ma, W.; Zhang, M.; Spaeth, E.L.; Wang, Y.; Battula, V.L.; Mak, P.Y.; Schallmoser, K.; et al. Reciprocal Leukemia-Stroma VCAM-1/VLA-4-Dependent Activation of NF-ΚB Mediates Chemoresistance. Blood 2014, 123, 2691–2702.
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-ΚB Pathway for the Therapy of Diseases: Mechanism and Clinical Study. Signal Transduct. Target. Ther. 2020, 5, 209.
- Park, M.H.; Hong, J.T. Roles of NF-ΚB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15.
- Vlahopoulos, S.A. Aberrant Control of NF-ΚB in Cancer Permits Transcriptional and Phenotypic Plasticity, to Curtail Dependence on Host Tissue: Molecular Mode. Cancer Biol. Med. 2017, 14, 254–270.
- Puissant, A.; Medyouf, H. Walking the Tightrope: Balancing Delicate Inflammation Response to Eradicate Acute Myeloid Leukemia. Cancer Discov. 2022, 12, 1617–1619.
- Ellegast, J.M.; Alexe, G.; Hamze, A.; Lin, S.; Uckelmann, H.J.; Rauch, P.J.; Pimkin, M.; Ross, L.S.; Dharia, N.V.; Robichaud, A.L.; et al. Unleashing Cell-Intrinsic Inflammation as a Strategy to Kill AML Blasts. Cancer Discov. 2022, 12, 1760–1781.
- Volk, A.; Li, J.; Xin, J.; You, D.; Zhang, J.; Liu, X.; Xiao, Y.; Breslin, P.; Li, Z.; Wei, W.; et al. Co-Inhibition of NF-ΚB and JNK Is Synergistic in TNF-Expressing Human AML. J. Exp. Med. 2014, 211, 1093–1108.
- Csizmar, C.M.; Kim, D.-H.; Sachs, Z. The Role of the Proteasome in AML. Blood Cancer J. 2016, 6, e503.
- Kagoya, Y.; Yoshimi, A.; Kataoka, K.; Nakagawa, M.; Kumano, K.; Arai, S.; Kobayashi, H.; Saito, T.; Iwakura, Y.; Kurokawa, M. Positive Feedback between NF-ΚB and TNF-α Promotes Leukemia-Initiating Cell Capacity. J. Clin. Investig. 2014, 124, 528–542.
- Li, J.; Volk, A.; Zhang, J.; Cannova, J.; Dai, S.; Hao, C.; Hu, C.; Sun, J.; Xu, Y.; Wei, W.; et al. Sensitizing Leukemia Stem Cells to NF-ΚB Inhibitor Treatment in Vivo by Inactivation of Both TNF and IL-1 Signaling. Oncotarget 2016, 8, 8420–8435.
- Grondona, P.; Bucher, P.; Schulze-Osthoff, K.; Hailfinger, S.; Schmitt, A. NF-ΚB Activation in Lymphoid Malignancies: Genetics, Signaling, and Targeted Therapy. Biomedicines 2018, 6, 38.
- Xiao, G.; Fu, J. NF-ΚB and Cancer: A Paradigm of Yin-Yang. Am. J. Cancer Res. 2011, 1, 192–221.
- Fang, J.; Rhyasen, G.; Bolanos, L.; Rasch, C.; Varney, M.; Wunderlich, M.; Goyama, S.; Jansen, G.; Cloos, J.; Rigolino, C.; et al. Cytotoxic Effects of Bortezomib in Myelodysplastic Syndrome/Acute Myeloid Leukemia Depend on Autophagy-Mediated Lysosomal Degradation of TRAF6 and Repression of PSMA1. Blood 2012, 120, 858–867.
- Hosseini, M.M.; Kurtz, S.E.; Abdelhamed, S.; Mahmood, S.; Davare, M.A.; Kaempf, A.; Elferich, J.; McDermott, J.E.; Liu, T.; Payne, S.H.; et al. Inhibition of Interleukin-1 Receptor-Associated Kinase-1 Is a Therapeutic Strategy for Acute Myeloid Leukemia Subtypes. Leukemia 2018, 32, 2374–2387.
- Bosman, M.C.J.; Schepers, H.; Jaques, J.; Brouwers-Vos, A.Z.; Quax, W.J.; Schuringa, J.J.; Vellenga, E. The TAK1-NF-ΚB Axis as Therapeutic Target for AML. Blood 2014, 124, 3130–3140.
- Rhyasen, G.W.; Bolanos, L.; Starczynowski, D.T. Differential IRAK Signaling in Hematologic Malignancies. Exp. Hematol. 2013, 41, 1005–1007.
- Sawaguchi, Y.; Yamazaki, R.; Nishiyama, Y.; Mae, M.; Abe, A.; Nishiyama, H.; Nishisaka, F.; Ibuki, T.; Sasai, T.; Matsuzaki, T. Novel Pan-Pim Kinase Inhibitors with Imidazopyridazine and Thiazolidinedione Structure Exert Potent Antitumor Activities. Front. Pharmacol. 2021, 12, 672536.
- Liu, Z.; Han, M.; Ding, K.; Fu, R. The Role of Pim Kinase in Immunomodulation. Am. J. Cancer Res. 2020, 10, 4085–4097.
- Wang, Y.; Xiu, J.; Ren, C.; Yu, Z. Protein Kinase PIM2: A Simple PIM Family Kinase with Complex Functions in Cancer Metabolism and Therapeutics. J. Cancer 2021, 12, 2570–2581.
- Nihira, K.; Ando, Y.; Yamaguchi, T.; Kagami, Y.; Miki, Y.; Yoshida, K. Pim-1 Controls NF-B Signalling by Stabilizing RelA/P65. Cell Death Differ. 2010, 17, 689–698.
- Zhu, N.; Ramirez, L.M.; Lee, R.L.; Magnuson, N.S.; Bishop, G.A.; Gold, M.R. CD40 Signaling in B Cells Regulates the Expression of the Pim-1 Kinase via the NF-ΚB Pathway. J. Immunol. 2002, 168, 744–754.
- Filippakopoulos, P.; Knapp, S. Targeting Bromodomains: Epigenetic Readers of Lysine Acetylation. Nat. Rev. Drug Discov. 2014, 13, 337–356.
- Hajmirza, A.; Emadali, A.; Gauthier, A.; Casasnovas, O.; Gressin, R.; Callanan, M.B. BET Family Protein BRD4: An Emerging Actor in NFκB Signaling in Inflammation and Cancer. Biomedicines 2018, 6, 16.
- Huang, B.; Yang, X.-D.; Zhou, M.-M.; Ozato, K.; Chen, L.-F. Brd4 Coactivates Transcriptional Activation of NF-KappaB via Specific Binding to Acetylated RelA. Mol. Cell. Biol. 2009, 29, 1375–1387.
- Zou, Z.; Huang, B.; Wu, X.; Zhang, H.; Qi, J.; Bradner, J.; Nair, S.; Chen, L.-F. Brd4 Maintains Constitutively Active NF-ΚB in Cancer Cells by Binding to Acetylated RelA. Oncogene 2014, 33, 2395–2404.
- Smale, S.T. Hierarchies of NF-ΚB Target-Gene Regulation. Nat. Immunol. 2011, 12, 689–694.
- Zuber, J.; Shi, J.; Wang, E.; Rappaport, A.R.; Herrmann, H.; Sison, E.A.; Magoon, D.; Qi, J.; Blatt, K.; Wunderlich, M.; et al. RNAi Screen Identifies Brd4 as a Therapeutic Target in Acute Myeloid Leukaemia. Nature 2011, 478, 524–528.
- Brown, J.D.; Lin, C.Y.; Duan, Q.; Griffin, G.; Federation, A.; Paranal, R.M.; Bair, S.; Newton, G.; Lichtman, A.; Kung, A.; et al. NF-ΚB Directs Dynamic Super Enhancer Formation in Inflammation and Atherogenesis. Mol. Cell 2014, 56, 219–231.
- Nicodeme, E.; Jeffrey, K.L.; Schaefer, U.; Beinke, S.; Dewell, S.; Chung, C.-W.; Chandwani, R.; Marazzi, I.; Wilson, P.; Coste, H.; et al. Suppression of Inflammation by a Synthetic Histone Mimic. Nature 2010, 468, 1119–1123.
- Gu, L.; Findley, H.W.; Zhou, M. MDM2 Induces NF-KappaB/P65 Expression Transcriptionally through Sp1-Binding Sites: A Novel, P53-Independent Role of MDM2 in Doxorubicin Resistance in Acute Lymphoblastic Leukemia. Blood 2002, 99, 3367–3375.
- Thomasova, D.; Mulay, S.R.; Bruns, H.; Anders, H.-J. P53-Independent Roles of MDM2 in NF-ΚB Signaling: Implications for Cancer Therapy, Wound Healing, and Autoimmune Diseases. Neoplasia 2012, 14, 1097–1101.
- Hayashi, Y.; Goyama, S.; Liu, X.; Tamura, M.; Asada, S.; Tanaka, Y.; Fukuyama, T.; Wunderlich, M.; O’Brien, E.; Mizukawa, B.; et al. Antitumor Immunity Augments the Therapeutic Effects of P53 Activation on Acute Myeloid Leukemia. Nat. Commun. 2019, 10, 4869.
- Kuhn, D.J.; Orlowski, R.Z. The Immunoproteasome as a Target in Hematologic Malignancies. Semin. Hematol. 2012, 49, 258–262.
- Ma, W.; Kantarjian, H.; Bekele, B.; Donahue, A.C.; Zhang, X.; Zhang, Z.J.; O’Brien, S.; Estey, E.; Estrov, Z.; Cortes, J.; et al. Proteasome Enzymatic Activities in Plasma as Risk Stratification of Patients with Acute Myeloid Leukemia and Advanced-Stage Myelodysplastic Syndrome. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 3820–3826.
- Niewerth, D.; Dingjan, I.; Cloos, J.; Jansen, G.; Kaspers, G. Proteasome Inhibitors in Acute Leukemia. Expert Rev. Anticancer Ther. 2013, 13, 327–337.
- Bonardi, F.; Fusetti, F.; Deelen, P.; van Gosliga, D.; Vellenga, E.; Schuringa, J.J. A Proteomics and Transcriptomics Approach to Identify Leukemic Stem Cell (LSC) Markers. Mol. Cell. Proteom. MCP 2013, 12, 626–637.
