Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Lamiaa M.A. Ali and Version 3 by Vivi Li.
In the race to design ever more effective therapy with ever more focused and controlled actions, nanomedicine and phototherapy seem to be two allies of choice. Indeed, the use of nanovectors making it possible to transport and protect genetic material is becoming increasingly important. In addition, the use of a method allowing the release of genetic material in a controlled way in space and time is also a strategy increasingly studied thanks to the use of lasers. In parallel, the use of interfering RNA and, more particularly, of small-interfering RNA (siRNA) has demonstrated significant potential for gene therapy.
- nanovectors
- photochemical internalization
- siRNA
- cancer
1. Introduction on Cancer and Treatments
Currently, cancer stands out as the first cause of death in the world after heart disease [1]. The increase in aging and population, as well as the changes in the distribution of the main risk factors, lead to rapid growth in cancer incidence and mortality. In 2020, 19.3 million new cases worldwide were identified, a number that is expected to increase to 28.4 million cases in 2040 [2].
Surgery, chemotherapy, radiotherapy, and hormone therapy are the main commonly used treatments despite the limitations of the specificity toward cancerous tissues, which lead to the key setbacks in cancer therapy as metastasis, tumor recurrence, and resistance to the treatments [3]. Therefore, there is an urgent need to develop new strategies to effectively kill cancer cells with little or no damage to healthy tissue.
Nanomedicine opens new hopes in solving many medical problems by developing several nanomaterials of organic or inorganic natures. The intrinsic properties of these nanomaterials, such as their nanometric size and large surface-to-volume ratio, open up many possibilities to explore their potential for the biomedical applications, especially for drug delivery, overcoming the chemotherapy limitations as systemic toxicity and multi-drug resistance mechanisms (MDR) [4].
Nowadays, several nanomedicines, a term that includes all nanomaterials used for biomedical applications [5], such as liposomes and albumin-based nanoparticles, are clinically approved for the treatment of cancer. Many others are in clinical trials and show great promises such as chemotherapy delivery systems, hyperthermia agents, and genetic or ribonucleic acid interference (RNAi) delivery systems [6].
2. Ribonucleic Acid Interference (RNAi) Technology
RNAi is a natural mechanism in eukaryotes for post-transcriptional gene silencing through (i) chromatin remodeling, (ii) inhibition of protein translation, or (iii) direct degradation of messenger RNA (mRNA) [7]. It was first discovered in 1998 by Fire and Mello research on Caenorhabditis elegans [8] and it serves as epigenetic regulator and defense mechanism against exogenous genes (e.g., viral or bacterial genes) and endogenous genes (e.g., transposons) [9][10][11][9,10,11]. In addition, it is considered as a promising strategy for treatment of cancer, primarily by specifically targeting key molecules involved in the molecular pathways of carcinogenesis [12][13][12,13]. RNAi mediates its action through non-coding short double-stranded RNA (nc-sdRNA) such as small-interfering RNA (siRNA) and microRNAs (miRNA). Single miRNA can inhibit the expression of several target genes simultaneously; however, to trigger gene silencing; siRNA is considered more efficient and specific than miRNA [14]. Here, rwesearchers focus on siRNA; thus, a description of the mechanism of action, siRNA-based cancer therapies, and barriers to siRNA delivery will be discussed in the following paragraphs.2.1. Mechanism of Action of siRNA
The biogenesis of siRNA starts with the presence of long dsRNA, which originates from different sources (e.g., viral, bacterial and synthetic RNA) in the cytoplasm (Figure 1). An enzyme called Dicer, a dsRNA-specific endoribonuclease from the RNase III protein family, cleaves the long dsRNA to about 21 nucleotides (nt) dsRNA called siRNA with 19 nt of complementary bases and a 2-nt overhang at each 3′-end. Afterwards, the formed siRNA duplex is loaded into a multiprotein RNA-induced silencing complex (RISC), in which a catalytic engine called the Argonaut protein (Ago-2) cleaves the passenger strand, keeping the active RISC with the guide strand. The siRNA guide strand recruits the RISC to complementary sequences in target mRNAs. A perfect siRNA base-pairing with mRNA causes direct mRNA cleavage by the catalytic RNase H domain of Ago-2, resulting in gene silencing, an effect that could last up to 7 days in rapidly divided cells and several weeks in nondividing cells [15][16][15,16].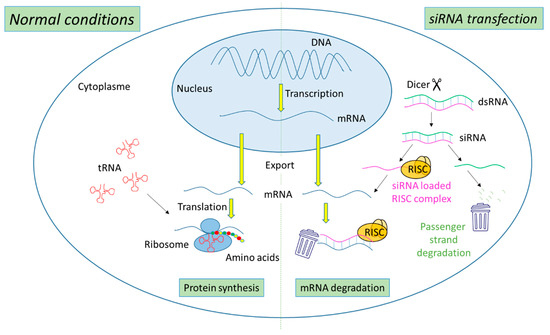
Figure 1. Representation of gene expression leading to protein synthesis in “normal conditions” in comparison with mechanism leading to mRNA degradation before protein synthesis in the presence of siRNA.
2.2. siRNA-Based Cancer Therapies
Recently, siRNA has emerged as a promising therapy for the treatment of several disorders, including cancer [17][18][17,18]. Its essential therapeutic strategy stems from its ability to suppress oncogenes and mutated tumor suppressor genes, as well as genes involved in MDR mechanism, resulting in the sensitization of cancer cells to treatment [19][20][19,20]. Anticancer siRNA targets can be categorized into (i) molecules involved in carcinogenesis, including molecules involved in oncogenic pathways, regulation of cell cycle, and apoptosis pathway; (ii) molecules involved in tumor–host interaction such as in cell adhesion, tumor extracellular matrix, tumor immune evasion, angiogenesis, invasion, and metastasis; and (iii) molecules participated in tumor resistance to chemotherapy, such as MDR and DNA repair proteins [14]. The first human clinical trial of siRNA encapsulated in targeted cyclodextrin polymer-based nanoparticles (CALAA-01) was started in 2008 by Calando Pharmaceuticals (Pasadena, CA, USA) for solid tumor cancer treatment. This phase I study was terminated in 2012 [21]. Table 1 summarizes siRNA-based cancer therapeutics in clinical trials.Table 1.
Anticancer siRNA-based therapeutics in clinical trials.
| Name/Sponsor | Route of Administration | Delivery System | Targeting Moiety | Target Gene | Disease | Clinical Trail Number (ClinicalTrials.gov) | Phase/Status | Period | Ref | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CALAA-01/Calando Pharmaceuticals | i.v. | Cyclodextrin polymer-based nanoparticle | Transferrin | RRM2 | Solid tumors (Melanoma, gastrointestinal, prostate, etc.) | NCT00689065 | Phase I/Terminated | 2008–2012 | [21] | ||
| siG12D LODER/Silenseed Ltd. | Endoscopic intervention | Biodegradable Polymeric matrix | ----- | KRAS(G12D) and G12X mutations | Locally advanced pancreatic cancer | NCT01188785 | Phase I/Completed | 2011–2013 | [22] | ||
| siG12D-LODERs plus chemotherapy (Gemcitabine + nab-Paclitaxel or Folfirinox or modified Folfirinox) /Silenseed Ltd. | Endoscopic intervention | Biodegradable Polymeric matrix | ----- | KRAS(G12D) and G12X mutations | Locally advanced pancreatic cancer | NCT01676259 | Phase II/Recruiting | 2018–Est.2023 | [23] | ||
| ALN-VSP02/Alnylam Pharmaceuticals | i.v. | Lipid nanoparticle | ----- | VEGF KSP |
Solid tumors with liver involvement. | NCT00882180 NCT01158079 |
Phase I/Completed | 2009–2011 2010–2012 |
[24] | ||
| TKM-PLK1 (TKM-080301)/National Cancer Institute (NCI) | Hepatic Intra-Arterial Administration | Lipid nanoparticle | ----- | PLK1 | Primary or secondary liver cancer. | NCT01437007 | Phase I/Completed | 2011–2012 | [25] | ||
| Arbutus Biopharma Corporation | i.v. | Cancer, neuroendocrine tumors, adrenocortical carcinoma | NCT01262235 | Phase I/II/Completed | 2010–2015 | ||||||
| Arbutus Biopharma Corporation | i.v. | Hepatocellular Carcinoma | NCT02191878 | Phase I/II/Completed | 2014–2016 | ||||||
| DCR-MYC/Dicerna Pharmaceuticals, Inc. | i.v. | EnCore | TM | lipid nanoparticle | ----- | MYC | Solid tumors, multiple myeloma, lymphoma | NCT02110563 | Phase I/Terminated | 2014–2016 | [26] |
| NBF-006/Nitto BioPharma, Inc. | Lipid nanoparticle | GSTP | Non-Small cell lung, pancreatic and colorectal Cancers | NCT03819387 | Phase I/Recruiting | 2019–Est.2023 | [27] | ||||
| Atu027/Silence Therapeutics GmbH | i.v. | Liposomes | ----- | PKN3 | Advanced Solid Cancer | NCT00938574 | Phase I/Completed | 2009–2012 | [28] | ||
| Atu027-I-02 (Atu027 plus gemcitabine)/Silence Therapeutics GmbH | i.v. | Liposomes | ----- | PKN3 | Advanced or Metastatic Pancreatic Cancer | NCT01808638 | Phase I/II/Completed | 2013/2016 | [29] | ||
| EphA2-targeting DOPC-encapsulated siRNA/M.D. Anderson Cancer Center | i.v. | Liposomes | ----- | EphA2 | Advanced or recurrent solid tumors | NCT01591356 | Phase I/Active, not recruiting | 2015–Est.2024 | [30] | ||
| Mesenchymal Stromal Cells-derived Exosomes with KRAS(G12D) siRNA/M.D. Anderson Cancer Center | MSC exosome | CD47 | KRAS(G12D) | Metastatic pancreatic ductal adenocarcinoma with KrasG12D mutation | NCT03608631 | Phase I/Recruiting | 2021–Est.2023 | [31] |
RRM2: M2 subunit of ribonucleotide reductase; VEGF: vascular endothelial growth factor; KSP: kinesin spindle protein; PLK1: Polo-like kinase 1; PKN3: protein kinase N3; MYC: name of oncogene; DCR-MYC: anti-MYC DsiRNA formulated in EnCore lipid nanoparticles; EphA2: ephrin type-A receptor 2; DOPC: 1,2-dioleoyl-sn-glycero-3-phosphatidylcholine; KRAS(G12D): oncongene; MSC: mesenchymal stem cells; GSTP: glutathione-S-transferase P.
So far, only four non-cancer related siRNA-based therapeutics are approved by the Food and Drug administration (FDA), which are Patisiran, Givosiran, Lumasiran, and Inclisiran branded as ONPATTRO®, GIVLAARI®, OXLUMO®, and LEQVIO®, respectively, by Alnylam Pharmaceuticals (Cambridge, MD, USA) [32].
2.3. Hurdles to siRNA Delivery
The in vitro and in vivo delivery of “naked” siRNA, without a delivery system, can come up against several extracellular and intracellular obstacles such as the rapid degradation by nucleases (t½ ~ 10 min), rapid renal clearance, activation of the innate immune system, and the low accumulation in the target organ after systemic administration. Moreover, siRNA is characterized not only by low cellular uptake due to its negative charge and high molecular weight (~13 kDa) but also by its inability to escape from the endo-lysosomal compartments to the cytoplasm [33][34][33,34]. Thus, to circumvent these drawbacks two approaches are commonly used. The first approach is the chemical modification of the phosphate backbone, the heterocyclic nucleobase, or the ribose sugar moiety in order to increase siRNA stability, affinity, and specificity toward targets [35]. Three of the four FDA-approved siRNA therapeutics (Givosiran, Lumasiran and Inclisiran) are composed of chemically modified siRNA conjugated to trivalent N-acetylgalactosamine (GalNAc), a ligand to asialoglycoprotein receptor (ASGPR), resulting in hepatocyte-specific delivery. These GalNAc conjugates are fully modified at the 2′ position of the ribose sugar with 2′-O-methyl (2′-OMe) or 2′-deoxy-2′-fluoro (2′-F) as well as including phosphorothioate linkages. Unfortunately, chemical modifications are associated with several limitations, such as toxicity and low biological activity [36][37][36,37]. The second approach is the incorporation of siRNA into delivery systems to ensure efficient and safe administration of siRNA to the target site. For years, viral vectors have been used for siRNA delivery due to their strong efficiency, but they raise safety concerns due to their high immunogenicity and carcinogenic effects [38]. On the contrary, nanomaterials are considered as potential candidates for siRNA delivery showing low immunogenicity and toxicity, ease preparation, and high loading capacity. Additionally, the cargo is protected from degradation and nanomaterials can be active- or passive-targeted delivery systems, stimuli-responsive release systems, and co-delivery systems of different drugs simultaneously. The first FDA-approved siRNA therapeutic, Patisiran, is composed of multicomponent lipid nanoparticles (LNP) encapsulating partially chemically modified siRNA, in which some of the nucleotides are chemically modified at 2′-OMe. These chemical modifications reduce the nuclease degradation and innate immune system stimulation, while LNP provides the liver-specific delivery of siRNA via apolipoprotein E (ApoE) receptor endocytosis aside from nuclease protection [39]. In general, nanomaterials are internalized in the cells by either nonendocytic or endocytic route depending on several factors such as nanomaterials physicochemical properties (e.g., size, shape, and charge); targeting moieties; etc. [40]. According to the mechanism of internalization, the fate of the nanomaterials inside cells is determined, for example if nanomaterials are internalized by clathrin-mediated endocytosis, then they will be trapped in the endosomes, which subsequently fuse with lysosomes and degradation will take place due to severe acidic conditions [4]. Therefore, the endo-lysosomal escape of nanomaterials for efficient cytosolic delivery of siRNA is mandatory in order to accomplish its biological activity. Several strategies have been developed to enhance the cytosolic delivery of siRNA [41] such as proton sponge effect [42], fusogenic groups [43], and photochemical internalization (PCI) technology. This rentryview focuses on the PCI mechanism for siRNA release and the next paragraphs will present a description of this mechanism with several examples of PCI-mediated cytosolic delivery of siRNA using different vectors.3. Photochemical Internalization (PCI) Mechanism
The PCI mechanism is a noninvasive technique that has developed over nearly two decades for multiple purposes including the treatment of cancer [44][45][44,45]. This technique is used to release macromolecules (peptides, proteins, and nucleic acids) confined in the endo-lysosomal compartments into the cytoplasm with the help of photosensitizers (PS) in light-dependent manner. Although, its similarity to photodynamic therapy (PDT) in components, including PS, oxygen, and light, differs from PDT in the final impact on cells. The PDT leads to cell death due to excessive production of reactive oxygen species (ROS), mainly singlet oxygen (1O2), which has a diffusion range of ~10–20 nm and t½ in µs [46][47][48][46,47,48]. While, PCI leads to disruption of endo-lysosomal membranes with no cytotoxic effect, as the accumulation of the PS in the endo-lysosomal membrane leads to local production of 1O2; hence, the damage is limited to its production zone [49]. The PCI process was first described by Berg K. et al. in 1999 [50] using several PS, including aluminum phtalocyanine disulfonate (AIPcS2a), in order to show their efficiency for the cytosolic delivery of plasmid encoding green fluorescent protein (GFP) into human colon cancer cells (HCT-116) and human melanoma cells (THX) after exposure to red light. In theis study, they established the concept of PCI as an ideal site-specific delivery tool that could be combined with other therapeutic modalities [50]. Two years later, Berg team showed the potential of PCI mechanism for in vivo applications using AlPcS2a for the PCI delivery of gelonin in tumor-bearing mice [51]. In addition, AlPcS2a-based PCI delivery of bleomycin in tumors has also been reported [52]. In 2009, the first-in-man dose-escalating trial of PCI for bleomycin delivery in patients with different types of solid malignancies has started (phase 1, NCT00993512, ClinicalTrials.gov). The trial ended with the results demonstrating the safety of the photosensitizer used for PCI, which is Amphinex, a disulfonate tetraphenyl chlorin (TPCS2a) illuminated by 652-nm laser light with an energy of 60 J/cm2 [53]. Here, several siRNA vectors of different natures (lipid-based, polymer-based, peptide-based, and nanoparticles), which release their cargo under PCI mechanism, will be discussed (Figure 2).
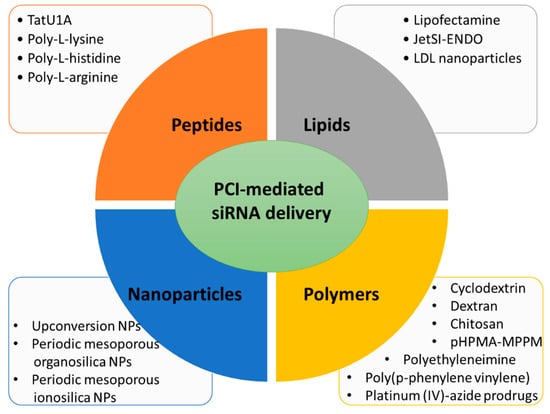
Figure 2. Main types of carriers used for PCI-mediated siRNA delivery discussed in this entry.
Main types of carriers used for PCI-mediated siRNA delivery discussed in this review.
