Oxygen is a unique molecule that possesses a spin quantum number S=1. In the condensed phases of oxygen, the delicate balance between the antiferromagnetic interaction and van der Waals force results in the various phases with different crystal structures. By applying ultrahigh magnetic fields, the antiferromagnetic coupling between O2 molecules breaks, and novel high-field phase (θ phase) appears. Since oxygen is an important element for various (bio-)chemical reactions, the reorientation of O2 molecules could be an attractive mechanism for controlling the reactivity.
1. Introduction
The paramagnetic nature of oxygen was discovered by M. Faraday in 1848, before the dawn of quantum mechanics
[1]. He studied the magnetism of bubbles filled with various gases and found that only oxygen is attracted by a permanent magnet. Later on, quantum mechanics provided an explanation of its magnetism; the half-filled
π electrons with polarized spins result in the triplet ground state with the spin-quantum number
S=1. The magnetic susceptibility is enhanced at lower temperatures, following the Curie law, and
itwe can
be clearly observe
d the paramagnetism of liquid oxygen. When the distance between O
2 molecules becomes shorter in a solid state, the exchange interactions between O
2 molecules become relevant for the ground state. By decreasing temperature, three phases of solid oxygen,
γ,
β, and
α phases, appear.
I Here, we provide an overview of t
he has been usedmagnetic properties of these solid and liquid phases (see also reviews [2,3,4,5,6]).
In th
is resea
trch, we have used high magnetic fields in the search for novel phases of oxygen. Because of the competing energy scale between the van der Waals and exchange interactions, spin-lattice coupling plays an important role in solid oxygen. Indeed, solid oxygen is called a “spin-controlled crystal”, where the crystal structure depends on the magnetic ground state. Using non-destructive magnets, the magnetization and optical properties of solid and liquid oxygen have been investigated up to 50 T
[2][3][4][5][6][7][8][15,16,19,22,44,45,46]. However, the exchange field in solid oxygen is even higher than this field range, and no phase transition was detected. When the AFM order is destroyed by extremely high magnetic fields, a novel phase with a new crystal structure may appear
. In this paper, we review our exploration for the novel phases of solid and liquid oxygen under ultrahigh magnetic fields [47,48,49,50,51,52,53,54,55,56,57,58].
2. Solid and Liquid Oxygen
2.1. Magnetization
Figure 1Figure 2 shows the magnetization curves with the initial temperatures of T0=9 K (α phase) and 32 K (β phase). The red and blue lines show the magnetization curves obtained by using a non-destructive magnet [4][19]. The magnetization linearly increases up to 110 T and suddenly shows a metamagnetic transition at around 120 T. This is a clear sign of a high-field phase of solid oxygen, termed the θ phase. The experimental results show both α-θ and β-θ transitions occur in a similar field range. The magnetization in the field-down sweep shows an anomaly at around 70 T with a large hysteresis loop. The magnetization curve below 70 T follows the slope of the β phase (blue curve), although the initial phase is the α phase (red curve). This probably reflects the temperature increase after the α-θ transition, which causes considerable dissipation as a hysteresis loss [9][56]. The magnetization of the θ phase depends on the peak value of the magnetic field, which is related to the slow dynamics of the phase transition compared to the magnetic-field duration of ∼6 μs. To reach the saturation value of 2 μB, a longer pulse duration above the transition field (∼100 T) is needed.
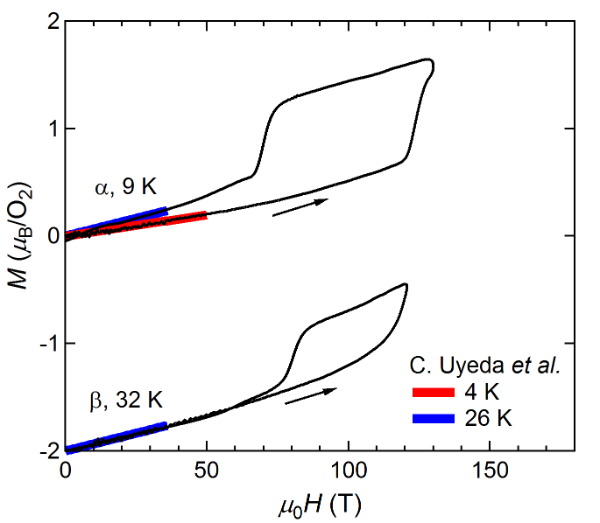
Figure 1. Magnetization curves of oxygen. (a) α and β phases. (b) γ and liquid phases. Magnetization curves obtained by using non-destructive magnets are shown for comparison [4]. The results for the β and liquid phases are shifted by −2 for clarity.
2.2. Optical Absorption
Figure 2Figure 3 shows the results of the optical-absorption measurements up to 127 T. Optical absorption around 2.16 eV is called as a bimolecular absorption, where two O
2 molecules are simultaneously excited with one photon absorption
[2][3][10][11][12][13][12,13,14,15,16,17]. At the top of the pulsed field (at 3
μs), the absorption spectra drastically change; the transmitted light intensity increases in all photon energy ranges, and the bimolecular absorption disappears. This clear anomaly corresponds to the
α-
θ transition of solid oxygen observed in the magnetization curve. Because of the increased magnetization, the probability of forming a singlet pair becomes almost zero, leading to the disappearance of the bimolecular absorption. The increase of the transmitted light in all photon energy ranges does not reflect the change in the bimolecular absorption, but rather, the light scattering. The samples used
in this study are polycrystals of solid oxygen. At the grain boundaries of polycrystalline solid oxygen, strong light scattering occurs because of the refractive-index mismatch. The light scattering is especially strong for the anisotropic phases (
α and
β phases), while it is relatively weak in the
γ phase because of the isotropic structure. The decrease of the light scattering in the
θ phase indicates that this phase also has isotropic optical properties.
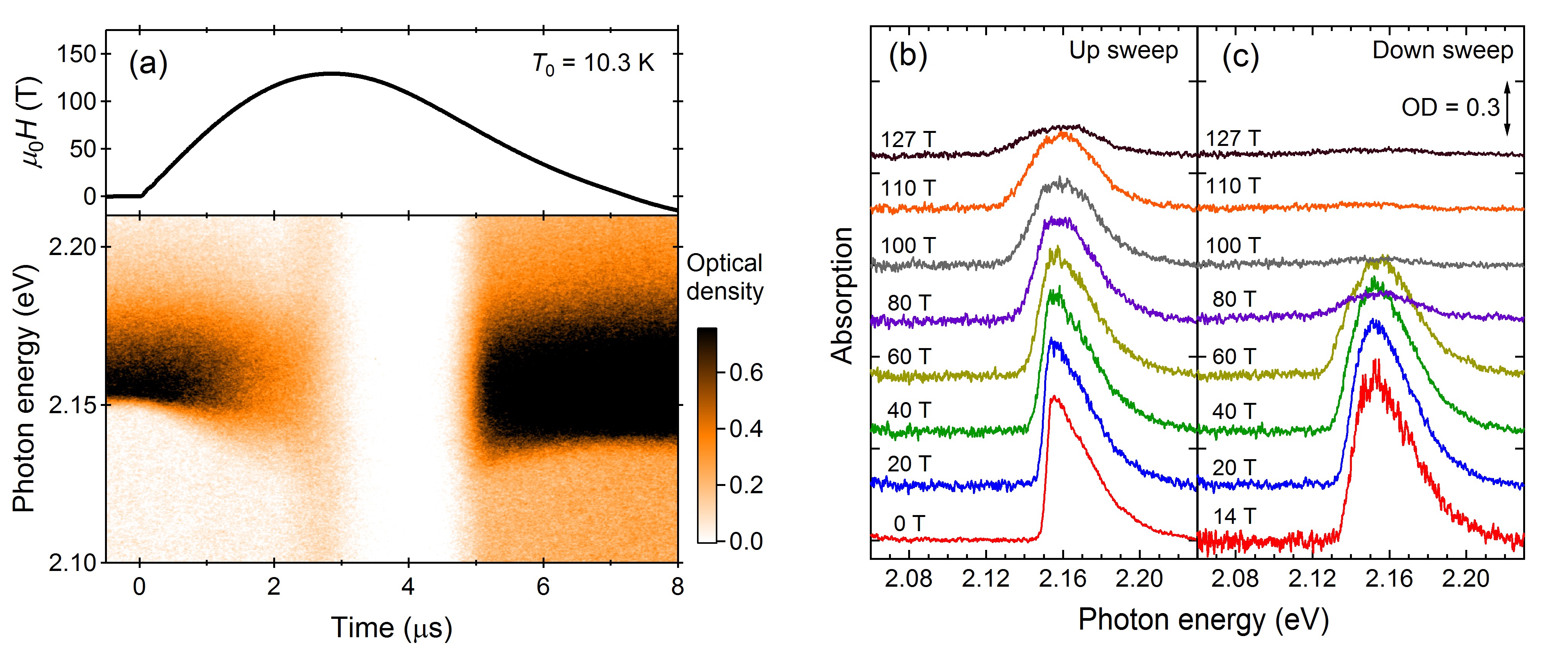
Figure 2. Results of the magneto-optical measurement on solid oxygen. (a) Magnetic field waveform and absorption spectra as a function of time. Optical density (OD) is shown in the color scale. The initial temperature is 10.3 K. (b,c) Absorption spectra for the (b) field-up sweep and (c) field-down sweeps.
2.3. Phase diagram
ResearchWe
rs propose the phase diagram of solid oxygen as
Figure 3 4 by combining the results of magnetization, optical spectroscopy, and magnetocaloric-effect measurements. For details of the procedures including the corrections for the MCE and hysteresis, see
[14][52]. The presented crystal structures of the
α,
β, and
γ phases are based on
[15][16][18,25]. Based on the slope of the phase boundary and the observed properties,
we propose that the
θ phase is characterized by the (i) large magnetization, (ii) isotropic crystal structure, and (iii) small entropy. From the theoretical point of view, the first-principle calculations suggest that the
Pa3¯ cubic structure can have lower energy than the
α-phase structure for a polarized spin state
[17][78]. Indeed, this is an FCC-like close-packing structure, which is favored in terms of the van der Waals energy and electric quadrupole interaction, and various molecular solids (CO
2, N
2, and N
2O) take it as a ground state at ambient pressure
[18][79]. In this structure, the nearest neighbor configuration is close to the X geometry rather than the H geometry. Thus, the AFM interaction in this crystal structure is weakened from the
α phase. Therefore,
we propose th
at the
Pa3¯ cubic structure is a promising candidate for the
θ phase, satisfying all of the features of this high-field phase.
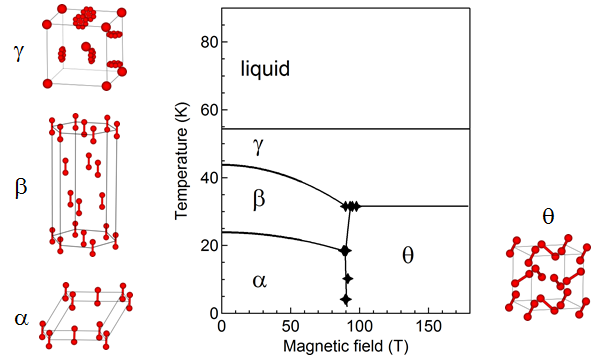
Figure 3. Proposed magnetic-field-temperature phase diagram of oxygen. The crystal structure of each phase is also shown.
3. Remaining Issues
3.1. Diffraction Experiment
The proposed crystal structure of the
θ phase is based on the indirect experimental observations and the theoretical calculations. To conclude the crystal structure,
rwe
searchers need to perform diffraction experiments for the
θ phase. Thanks to the recent development combining the free-electron X-ray laser and the portable STC system, diffraction experiments around 100 T are becoming accessible
[19][20][80,81]. In the near future, the direct determination of the crystal structure of the
θ phase might become possible.
3.2. High-Field Plastic and Liquid Phases
ItWe is propose
d the scenario of the field-induced molecular rearrangement of O
2 for all the condensed phases of oxygen because their local structure is an H-type configuration with AFM correlation. The only reliable results obtained for liquid oxygen are the ultrasound results at 77 K up to 90 T using a non-destructive magnet
[21][53]. The continuous softening and increasing acoustic attenuation up to 90 T suggest that the local molecular arrangement in liquid oxygen fluctuates under magnetic fields. The observed decrease of sound velocity reaches 8.5% at 90 T, which is quite large compared to other magnetic systems
[22][82]. In addition, the normalized acoustic attenuation coefficient (
α/f2) becomes 20 times larger than the zero-field value. Empirically, the value of
α/f2=200×10−15 s
2/m is extremely large for simple liquids
[23][83]. Since the dissipations due to the viscosity and thermal conductivity cannot explain the observed value, the anomalous acoustic attenuation suggests fluctuations of O
2 molecules
. We argue that this fluctuation could be related to the field-induced liquid–liquid transition or crossover, from a small-M to a large-M liquid.




