Salicylic acid (SA) is a very simple phenolic compound (a C7H6O3 compound composed of an aromatic ring, one carboxylic and a hydroxyl group) and this simplicity contrasts with its high versatility and the involvement of SA in several plant processes either in optimal conditions or in plants facing environmental cues, including heavy metal (HM) stress.
- metal toxicity
- ortho-hydroxybenzoic acid
- plant hormone
- metal pollution
- polyphenols
- signaling compound
1. Introduction
2. HM Stress and Its Impacts on Plants
Metals and metalloids with atomic density more than 6 g cm−3 are defined as (HM). Both, essential elements, micronutrients that are required in low concentration (e.g., Cu, Cr, Co, and Zn), and nonessential metals such as Pb, Cd, Hg, are incorporated in this group [48,49][48][49]. Increased concentration of both essential and nonessential elements is phytotoxic to flora and fauna [50,51][50][51]. Heavy metal contamination has become a serious environmental problem worldwide. The increased industrialization, injudicious population growth, and urbanization releases HM that compromise soil and water and pose harms to living biota due to their biomagnification through the food chain [52]. Natural activities such as eruption of volcano and erosion of rocks have contribute in increasing the release of toxic elements to the environment; however, increased human activities such as mining, painting, and refining have enhanced their concentration in the biosphere [53,54,55][53][54][55]. Soil pollution by HM poses serious concerns to the biotic and abiotic components of the ecosystem [56]. The increased amount of HM in soil leads to greater uptake by plants that can reduce plant growth, biomass, photosynthesis, crop yield, and quality in plant [57]. From a biological point of view, the top soil is the most active zone of soil that accumulates a large amount of toxic metals that poses serious concern to the environment [49,58,59][49][58][59]. The increased level of HM accumulation in plant organs negatively affects the cell metabolism in plants [60]. The different physiological activities in plants such as protein metabolism, photosynthesis, respiration, and morphogenesis are naturally affected by a high concentration of toxic compounds, such as HM [53,54,61,62][53][54][61][62]. For instance, Rascio et al. [63] documented a decreased root growth and altered morphogenesis in rice seedlings upon treatment with Cd. Many plant species such as Brassica napus, Helianthus annuus, Thalaspi caerulescens, Vigna radiata showed inhibition in photosynthesis in response to Cd treatment [64,65,66,67,68][64][65][66][67][68]. Recently, Tandon and Srivastava [69] investigated the Pb effect on the morphology and metabolism of Sesamum indicum and found that the increasing concentration of metal affected the growth of the plant. Further, the plant showed severe symptoms of chlorosis, necrosis and reduced chlorophyll, and protein content at higher doses of Pb [69]. The major outcome of metal toxicity is the peaked production of ROS due to impairment of photosynthetic process by HM [70]. ROS such as hydroxyl, superoxide, and hydrogen peroxide are produced as by-product during electron transport in photosynthesis and respiration pathways [71]. Under physiological conditions, ROS play a multitude of signaling roles in plants, as well as in other organisms and they take part in a finely-tuned and well-orchestrated regulatory network [72,73][72][73]. ROS are indeed integrated into a complex regulatory system in plants which encompasses ROS, plant hormones (e.g., ethylene (ET) and abscisic acid (ABA)), signaling molecules (e.g., salicylic acid (SA) and jasmonic acid (JA)), and secondary messengers (e.g., Ca2+) [74,75][74][75]. However, when ROS production exceeds the physiological levels, their accumulation can lead to oxidative stress in the cells, that cause lipids peroxidation, macromolecular degradation, membrane disruption, DNA breakage, and ion leakage in plants [70,74,75][70][74][75]. For instance, Kaur et al. [76] explored Pb-induced ultrastructural changes in roots of wheat and concluded that Pb inhibited root growth, caused ROS generation, and disrupted mitochondrial and nuclear integrity in the tested plant. The enhanced generation of ROS in the plant cell is controlled by a complex network of antioxidant machinery that maintains ROS homeostasis in the cell [77]. Plants have a finely-tuned and well-orchestrated defense system that includes enzymatic antioxidants such as catalase (CAT), superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione peroxidase (GPX) and glutathione reductase (GR), and nonenzymatic antioxidants such as ascorbic acid, glutathione, alkaloids, phenol compounds, and α-tocopherol for scavenging excessive ROS [49,61][49][61]. Moreover, phytohormones such as auxins, gibberellins, cytokinins, abscisic acid, ethylene, brassinosteroids, jasmonic acid, and SA take part in the defensive mechanism of plants against HM stress.3. Physiological Roles of SA in Plants Under HM Stress
Concerning the physiological role in plants, SA is known to play a pivotal role in regulating plant morphology, development, flowering, and stomatal closure [78,79][78][79]. SA also affects seedling germination, cell growth, and nodulation in legumes [80]. Khan et al. [81] reported increased leaf area and dry weight production in corn and soybean in response to SA. Furthermore, Hussein et al. [82] reported pot studies that documented improved growth, leaf number, dry biomass, and stem diameter in wheat plants when leaves were sprayed with SA. The rate of transpiration and stomatal index of plants increased in response to supplementation of SA [81]. The pigment concentration in wheat seeds significantly enhanced upon exposure to a low concentration (10−5 M) of SA. However, foliar application of SA reduced transpiration rate in test plants, Phaseolus vulgaris and Commelina communis which might be due to the SA-evoked stomatal closure [83,84,85,86,87][83][84][85][86][87]. Moreover, SA has been reported to increase the shelf life of cut flowers of rose and defer senescence by controlling water level in rose plants [86]. Plant growth regulators or phytohormones especially, gibberellins, auxin, cytokinins, ethylene, brassinosteroids, and also SA play a key role in providing HM tolerance in plants [83]. SA, a phenolic plant hormone, regulates photosynthesis, respiration, and antioxidant defense mechanism in plants under different abiotic stress such as high temperature, salinity, and HM [78,88,89][78][88][89]. SA pretreatment provides protection from various metals such as Pb, Hg, Cd, in different plants [90,91,92][90][91][92]. Supplementation of SA in combination with plant growth promoting bacteria reduces Cr-induced oxidative damage in maize by enhancing activities of antioxidant and nonantioxidant enzymes [93,94][93][94]. Earlier, Song et al. [95] reported SA mediated enhancement in the activities of CAT and SOD enzymes in barley leaves under Zn, Cu, and Mn stress. Further, carbohydrate metabolism in Cr-treated maize plants improved upon exposure to SA [94]. Alleviation of Cd toxicity was reported in mustard plants in response to exogenous treatment of SA [93]. Recently, SA treatment mitigated Cd stress in Brassica juncea plants and enhanced growth and photosynthesis in plants. Moreover, supplementation of SA reduced reactive oxygen species levels by strengthening the antioxidant defense system in plants and provides stability to the plant membrane [96]. The exogenous application of SA upregulates the antioxidant system, improves growth and yield, and results in lowering of oxidative damage under Pb stress in B. campestris [97]. A schematization of the protective role exerted by SA in HM-stressed plants is reported in Figure 2, whereas a literature survey on the effect of different HM on plant metabolism is reported in Table 1.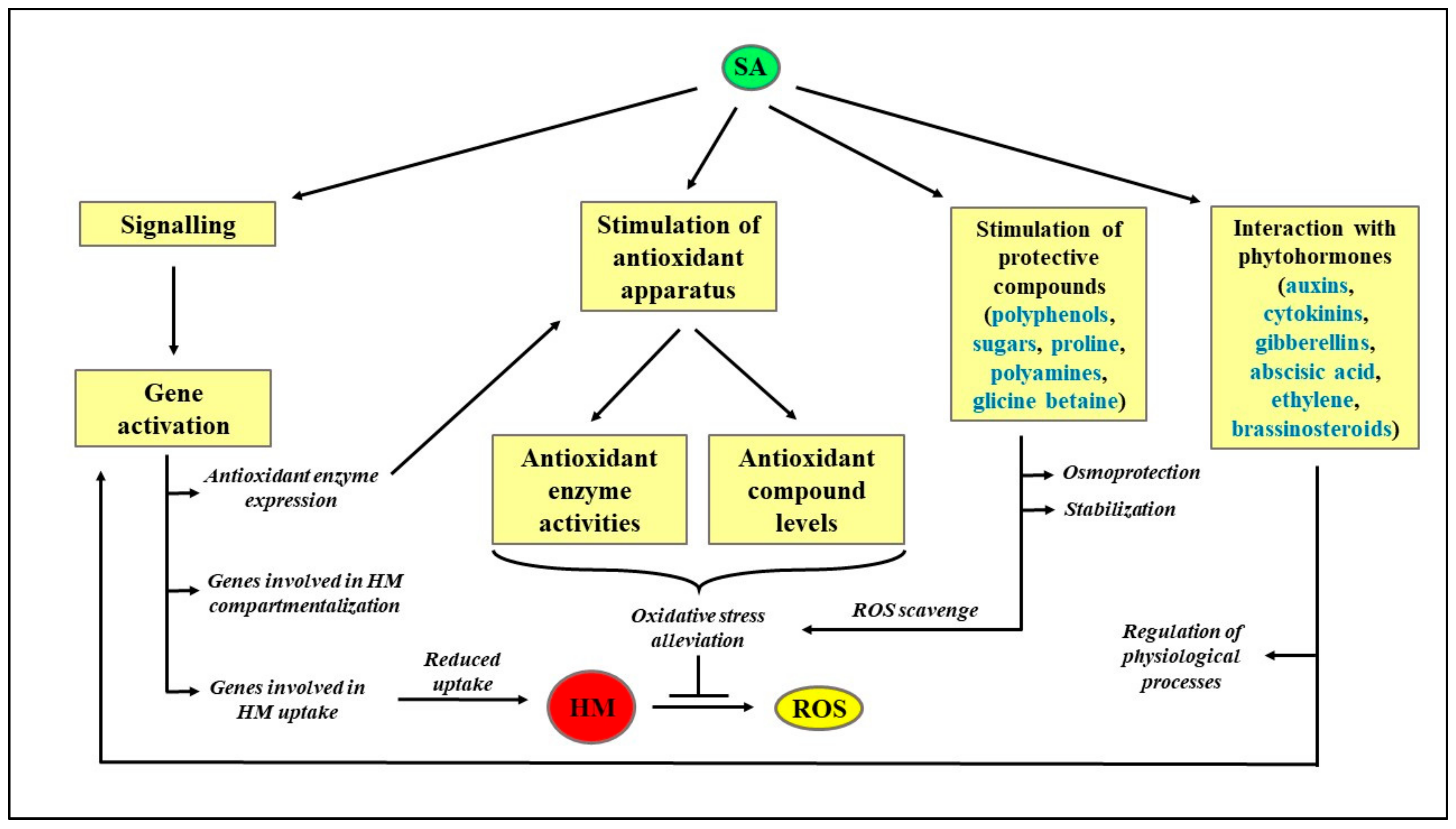
|
HM |
Species |
Effects of SA in plant metabolism |
References |
|---|---|---|---|
|
Cd |
Lemna minor L. |
Induced a reduction of Cd uptake, the maintenance of ionic homeostasis, improvement of PAL activity, activation of ROS scavenger and of the heat shock proteins. |
[98] |
|
Oryza sativa L. |
SA in association with NO reduced Cd uptake and accumulation, as well as ROS accumulation and malondialdehyde production through the maintenance of ascorbate and glutathione levels, and redox status. Improved the activities of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione S-transferase, and mono dehydroascorbate reductase. |
[41] |
|
|
Brassica juncea L. Czern |
Stimulating the stomatal activity and pore size, alleviated the inhibitory effect of Cd on photosynthesis. The Cd-generated oxidative burst was reduced via enhanced antioxidant activity (CAT and SOD) promoted by SA. |
[96] |
|
|
Nymphaea tetragona |
SA pretreatment decreased Cd concentration and increased the contents of glutathione, nonprotein thiol and phytochelatins. |
[99] |
|
|
Solanum tuberosum |
Cd stress increased endogenous SA level, relative water content, chlorophyll, and proline. Reduced lipid peroxidation, H2O2 and O2-. SA stimulated enzymatic antioxidants. |
[100] |
|
|
Triticum aestivum L. |
Induced a transient upregulation of protein kinases (SIPK). |
[101] |
|
|
Mentha piperita |
Improved photosynthesis by enhancing activity of RuBisCo and carbonic anhydrase. Reduced the oxidative stress by mitigating the production of free radicals by the maintenance of reduced glutathione pool and free radical scavenging enzymes. Furthermore, restored essential oils production previously affected by Cd. |
[102] |
|
|
Pb |
Brassica juncea L. Czern |
Co-application of 24-epibrassinolide and SA mitigates the negative effects of Pb, by lowering Pb metal uptake and enhancing the heavy metal tolerance index, antioxidative capacities, organic acid levels, phenolic content, water content, and relative water content. |
[37] |
|
Zea mays L. |
Improved nitrate reductase activity, glutathione content, and regulated the amino acids metabolism. |
[103] |
|
|
Triticum aestivum L. |
Suppressed chlorophyll degradation, electrolyte leakage, and malondialdehyde accumulation. Furthermore, enhanced the production of total soluble carbohydrates, proline, and the activities of SOD, CAT, and peroxidases. |
[104] |
|
|
Brassica campestris L. |
Improved plant growth and yield upregulating, in the antioxidant defense system, both enzymatic and nonenzymatic components. |
[93] |
|
|
Zea mays L. |
In combination with sodium hydrosulfide reduced arginine, proline, and methionine accumulation and increased nitric oxide and glycine betaine content. Moreover, it regulated the expression of ZmSAMD and ZmACS6 genes (genes involved in methionine metabolism). |
[105] |
|
|
As |
Trigonella foenum-graecum L. |
Enhanced root growth and increased protein content, free amino acids, and soluble sugars in both cotyledons and radicles. Moreover, it enhanced the activity of hydrolytic enzymes (α- and β-amylase). |
[106] |
|
Artemisia annua L. |
Increased endogenous SA level, reduced H2O2 and O2− generation, as well as lipid peroxidation. Reverted biomass and chlorophyll content. Increased artemisinin, and dihydroartemisinic acid level. Upregulated the expression of four key artemisinin biosynthetic pathway genes (CYP71AV1, ALDH1, ADS, and DBR2). |
[107] |
|
|
Artemisia annua L. |
Upregulated proteins related to energy metabolism, photosynthesis, secondary metabolism, transcriptional regulators, transport proteins, and proteins related to lipid metabolism. |
[108] |
|
|
Helianthus annuus L. |
Alleviated the negative effect of As on growth and decreased oxidative injuries through the increasing of the enzymatic activity of ROS scavengers such as CAT, ascorbate peroxidase (APX), and glutathione peroxidase, whereas the activity of SOD and guaiacol peroxidase activities was reduced. |
[109] |
|
|
Oryza sativa L. |
As enhanced endogenous level of SA and NO level through the enhancement of nitrate reductase activity. |
[110] |
|
|
Cr |
Sorghum bicolor L. |
Increased both APX and hydrogen peroxide content and decreased the peroxidase activity and ascorbic acid content. |
[111] |
|
Brassica napus L. |
Increased dry biomass, enhanced plant growth, and strengthened the reactive oxygen scavenging system by improving the activity in Cr-damaged organelles. |
[112] |
|
|
Oryza sativa L. |
Reduced the concentration and translocation of Cr in shoots but not in roots, suggesting a detoxification strategy based on Cr sequestration in roots. Increased growth parameters, membrane stability, and protein content. |
[113] |
|
|
Ni |
Brassica juncea L. Czern. & Coss. |
Restored growth and photosynthesis increasing the activities of enzymes associated with antioxidant systems, especially the glyoxalase system and the ascorbate–glutathione cycle (AsA–GSH) cycle. It had an additive effect on the activities of the ascorbate and glutathione pools, and the AsA–GSH enzymes and restored the content of mineral nutrient. |
[114] |
|
Eleusine coracana L. |
Inhibited Ni transport from roots to shoots, increased chlorophyll content, and the photosynthetic rate, increased the uptake of mineral content, reduced H2O2 and proline content, and enhanced the activity of antioxidant enzymes (SOD, CAT, APX). |
[115] |
|
|
Melissa officinalis L. |
Decreased Ni transport to the shoots, increased carotenoid content, induced a significant decrease in electrolyte leakage in stressed plants. |
[116] |
|
|
Alyssum inflatum Náyr. |
Mitigated Ni oxidative effects by reducing H2O2 concentration. Reversed the detrimental effects of Ni on carotenoid content and reduced the proline content. |
[117] |
|
|
Co |
Triticum aestivum L. |
Decreased the accumulation of H2O2 and MDA and improved the activity of antioxidant enzymes. |
[40] |
|
Cu |
Gossypium barbadense L. |
Limited Cu translocation and improved the activities of antioxidant enzymes. |
[118] |
|
Zea mays L. |
Lowered Cu and H2O2 accumulation in roots. Induced a reduction of MnSODII activity accompanied by a decrease in H2O2 concentration. |
[119] |
|
|
Zea mays L. |
Increased the biomass, root and shoot length, number and leaves area. |
[119] |
References
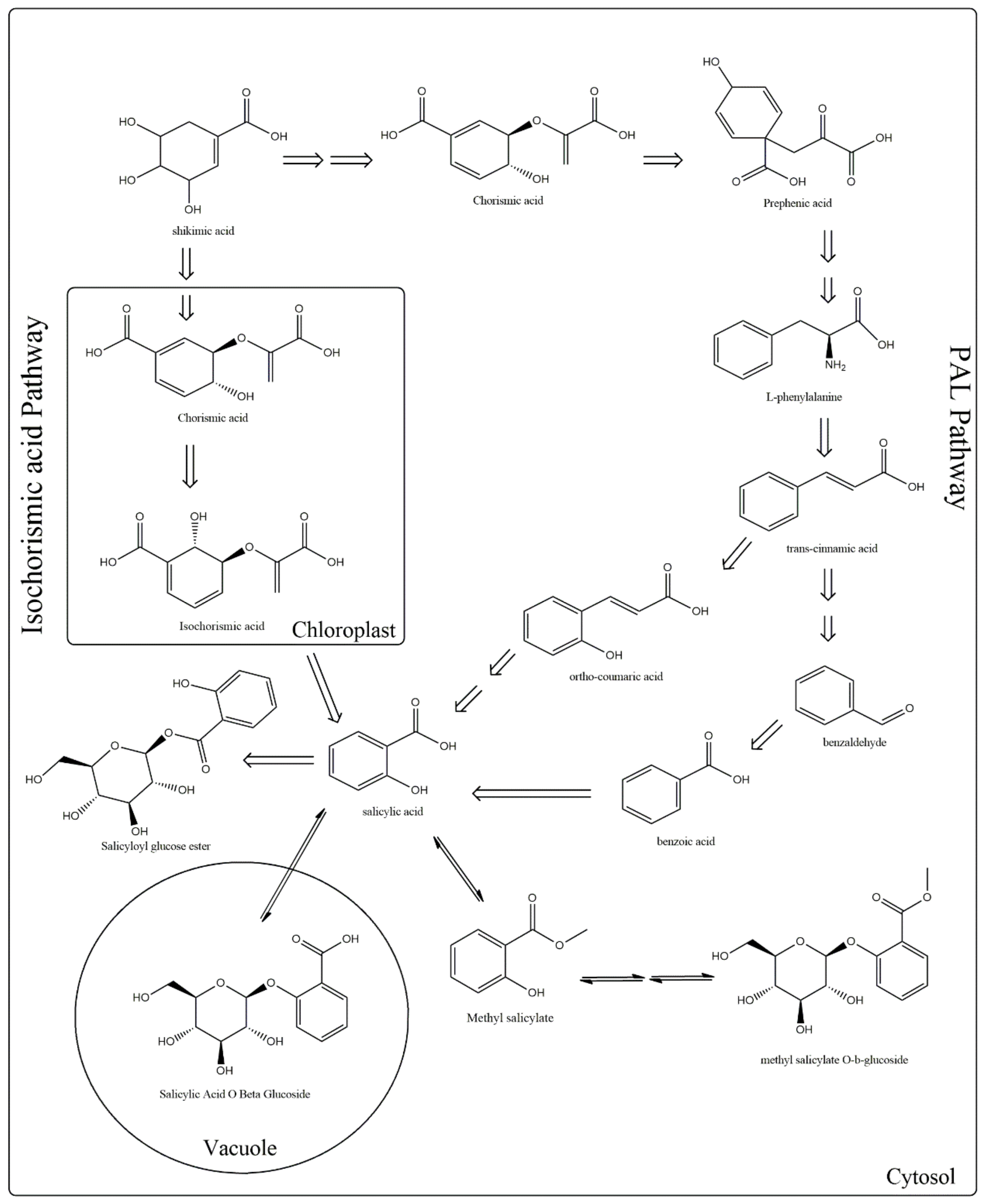
- Chen, Z.; Zheng, Z.; Huang, J.; Lai, Z.; Fan, B. Biosynthesis of salicylic acid in plants. Plant Sign. Behav. 2009, 4, 493–496.
- Wani, A.B.; Chadar, H.; Wani, A.H.; Singh, S.; Upadhyay, N. Salicylic acid to decrease plant stress. Environ. Chem. Lett. 2017, 15, 101–123.
- Lovelock, D.A.; Šola, I.; Marschollek, S.; Donald, C.E.; Rusak, G.; van Pée, K.H.; Ludwig-Müller, J.; Cahill, D.M. Analysis of salicylic acid-dependent pathways in Arabidopsis thaliana following infection with Plasmodiophora brassicae and the influence of salicylic acid on disease. Molecul. Plant Pathol. 2016, 17, 1237–1251.
- Maruri-López, I.; Aviles-Baltazar, N.Y.; Buchala, A.; Serrano, M. Intra and extracellular journey of the phytohormone salicylic acid. Front. Plant Sci. 2019, 10, 423.
- Raskin, I. Role of salicylic acid in plants. Annu. Rev. Plant Biol. 1992, 43, 439–463.
- Muthulakshmi, S.; Lingakumar, K. Role of salicylic acid (SA) in plants—A review. Int. J. Appl. Res. 2017, 3, 33–37.
- Foster, S. Tyler’s Honest Herbal: A Sensible Guide to the Use of Herbs and Related Remedies; Routledge: New York, NY, USA, 1999.
- Petrek, J.; Havel, L.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Kizek, R. Analysis of salicylic acid in willow barks and branches by an electrochemical method. Russ. J. Plant Physiol. 2007, 54, 553–558.
- Arif, H.; Aggarwal, S. Salicylic Acid (Aspirin); StatPearls Publishing LLC.: Tampa, FL, USA; St. Petersburg, Russia, 2019.
- Shine, M.; Yang, J.W.; El-Habbak, M.; Nagyabhyru, P.; Fu, D.Q.; Navarre, D.; Ghabrial, S.; Kachroo, P.; Kachroo, A. Cooperative functioning between phenylalanine ammonia lyase and isochorismate synthase activities contributes to salicylic acid biosynthesis in soybean. New Phytol. 2016, 212, 627–636.
- Zhang, Y.; Fu, X.; Hao, X.; Zhang, L.; Wang, L.; Qian, H.; Zhao, J. Molecular cloning and promoter analysis of the specific salicylic acid biosynthetic pathway gene phenylalanine ammonia-lyase (AaPAL1) from Artemisia annua. Biotech. Appl. Biochem. 2016, 63, 514–524.
- Chong, J.; Pierrel, M.-A.; Atanassova, R.; Werck-Reichhart, D.; Fritig, B.; Saindrenan, P. Free and conjugated benzoic acid in tobacco plants and cell cultures. Induced accumulation upon elicitation of defense responses and role as salicylic acid precursors. Plant Physiol. 2001, 125, 318–328.
- Hayat, S.; Ali, B.; Ahmad, A. Salicylic acid: Biosynthesis, metabolism and physiological role in plants. In Salicylic Acid: A Plant Hormone; Springer: Dordrecht, The Netherlands, 2007; pp. 1–14.
- Yalpani, N.; León, J.; Lawton, M.A.; Raskin, I. Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 1993, 103, 315–321.
- Silverman, P.; Seskar, M.; Kanter, D.; Schweizer, P.; Metraux, J.-P.; Raskin, I. Salicylic acid in rice (biosynthesis, conjugation, and possible role). Plant Physiol. 1995, 108, 633–639.
- Métraux, J.-P. Recent breakthroughs in the study of salicylic acid biosynthesis. Trends Plant Sci. 2002, 7, 332–334.
- Garcion, C.; Lohmann, A.; Lamodière, E.; Catinot, J.; Buchala, A.; Doermann, P.; Métraux, J.-P. Characterization and biological function of the Isochorismate Synthase2 gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287.
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 2019, 365, 498–502.
- Wei, Y.; Liu, G.; Chang, Y.; He, C.; Shi, H. Heat shock transcription factor 3 regulates plant immune response through modulation of salicylic acid accumulation and signalling in cassava. Mol. Plant Pathol. 2018, 19, 2209–2220.
- Hartmann, M.; Zeier, J. N-Hydroxypipecolic acid and salicylic acid: A metabolic duo for systemic acquired resistance. Curr. Opin. Plant Biol. 2019, 50, 44–57.
- El-Shazoly, R.M.; Metwally, A.A.; Hamada, A.M. Salicylic acid or thiamin increases tolerance to boron toxicity stress in wheat. J. Plant Nutr. 2019, 42, 702–722.
- Luo, J.; Xia, W.; Cao, P.; Xiao, Z.A.; Zhang, Y.; Liu, M.; Zhan, C.; Wang, N. Integrated transcriptome analysis reveals plant hormones jasmonic acid and salicylic acid coordinate growth and defense responses upon fungal infection in poplar. Biomolecules 2019, 9, 12.
- Pasternak, T.; Groot, E.P.; Kazantsev, F.V.; Teale, W.; Omelyanchuk, N.; Kovrizhnykh, V.; Palme, K.; Mironova, V.V. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiol. 2019, 180, 1725–1739.
- Cleland, C.F.; Ajami, A. Identification of the flower-inducing factor isolated from aphid honeydew as being salicylic acid. Plant Physiol. 1974, 54, 904–906.
- Raskin, I.; Skubatz, H.; Tang, W.; Meeuse, B.J. Salicylic acid levels in thermogenic and non-thermogenic plants. Ann. Bot. 1990, 66, 369–373.
- Dempsey, D.M.A.; Klessig, D.F. How does the multifaceted plant hormone salicylic acid combat disease in plants and are similar mechanisms utilized in humans? BMC Biol. 2017, 15, 23.
- Klessig, D.F.; Choi, H.W.; Dempsey, D.M.A. Systemic acquired resistance and salicylic acid: Past, present, and future. Mol. Plant-Microbe Interact. 2018, 31, 871–888.
- Subban, K.; Subramani, R.; Srinivasan, V.P.M.; Johnpaul, M.; Chelliah, J. Salicylic acid as an effective elicitor for improved taxol production in endophytic fungus Pestalotiopsis microspora. PLoS ONE 2019, 14, e0212736.
- Tripathi, D.; Raikhy, G.; Kumar, D. Chemical elicitors of systemic acquired resistance—Salicylic acid and its functional analogs. Curr. Plant Biol. 2019, 17, 48–59.
- Nadeem, M.; Ahmad, W.; Zahir, A.; Hano, C.; Abbasi, B.H. Salicylic acid-enhanced biosynthesis of pharmacologically important lignans and neo lignans in cell suspension culture of Linum ussitatsimum L. Eng. Life Sci. 2019, 19, 168–174.
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.-J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671.
- Safari, F.; Akramian, M.; Salehi-Arjmand, H.; Khadivi, A. Physiological and molecular mechanisms underlying salicylic acid-mitigated mercury toxicity in lemon balm (Melissa officinalis L.). Ecotoxic Environ. Safety 2019, 183, 109542.
- Dalvi, A.A.; Bhalerao, S.A. Response of plants towards heavy metal toxicity: An overview of avoidance, tolerance and uptake mechanism. Ann. Plant. Sci. 2013, 2, 362–368.
- Shi, G.; Cai, Q.; Liu, Q.; Wu, L. Salicylic acid-mediated alleviation of cadmium toxicity in hemp plants in relation to cadmium uptake, photosynthesis, and antioxidant enzymes. Acta Physiol. Plant 2009, 31, 969–977.
- Wang, C.; Zhang, S.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Lu, J.; Li, L. Salicylic acid involved in the regulation of nutrient elements uptake and oxidative stress in Vallisneria natans (Lour.) Hara under Pb stress. Chemosphere 2011, 84, 136–142.
- Wei, T.; Lv, X.; Jia, H.; Hua, L.; Xu, H.; Zhou, R.; Zhao, J.; Ren, X.; Guo, J. Effects of salicylic acid, Fe (II) and plant growth-promoting bacteria on Cd accumulation and toxicity alleviation of Cd tolerant and sensitive tomato genotypes. J. Environ. Manag. 2018, 214, 164–171.
- Kohli, S.K.; Handa, N.; Sharma, A.; Gautam, V.; Arora, S.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Combined effect of 24-epibrassinolide and salicylic acid mitigates lead (Pb) toxicity by modulating various metabolites in Brassica juncea L. seedlings. Protoplasma 2018, 255, 11–24.
- Guo, J.; Zhou, R.; Ren, X.; Jia, H.; Hua, L.; Xu, H.; Lv, X.; Zhao, J.; Wei, T. Effects of salicylic acid, Epi-brassinolide and calcium on stress alleviation and Cd accumulation in tomato plants. Ecotoxic. Environ. Saf. 2018, 157, 491–496.
- Malik, Z.A.; Lal, E.P.; Mir, Z.A.; Lone, A.H. Effect of salicyclic acid and indole acetic acid on tomato crop under induced salinity and cadmium stressed environment: A Review. Int. J. Plant Soil Sci. 2018, 26, 1–6.
- Mohamed, H.E.; Hassan, A.M. Role of salicylic acid in alleviating cobalt toxicity in wheat (Triticum aestivum L.) seedlings. J. Agric. Sci. 2019, 11.
- Mostofa, M.G.; Rahman, M.; Ansary, M.; Uddin, M.; Fujita, M.; Tran, L.-S.P. Interactive effects of salicylic acid and nitric oxide in enhancing rice tolerance to cadmium stress. Int. J. Mol. Sci. 2019, 20, 5798.
- Wang, Y.-Y.; Wang, Y.; Li, G.-Z.; Hao, L. Salicylic acid-altering Arabidopsis plant response to cadmium exposure: Underlying mechanisms affecting antioxidation and photosynthesis-related processes. Ecotoxicol. Environ. Safety 2019, 169, 645–653.
- Belkadhi, A.; De Haro, A.; Obregon, S.; Chaïbi, W.; Djebali, W. Positive effects of salicylic acid pretreatment on the composition of flax plastidial membrane lipids under cadmium stress. Environ. Sci. Poll. Res. 2015, 22, 1457–1467.
- Tamás, L.; Mistrík, I.; Alemayehu, A.; Zelinová, V.; Bočová, B.; Huttová, J. Salicylic acid alleviates cadmium-induced stress responses through the inhibition of Cd-induced auxin-mediated reactive oxygen species production in barley root tips. J. Plant Physiol. 2015, 173, 1–8.
- Cui, W.; Li, L.; Gao, Z.; Wu, H.; Xie, Y.; Shen, W. Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J. Exp. Bot. 2012, 63, 5521–5534.
- Tahjib-Ul-Arif, M.; Siddiqui, M.N.; Sohag, A.A.M.; Sakil, M.A.; Rahman, M.M.; Polash, M.A.S.; Mostofa, M.G.; Tran, L.-S.P. Salicylic acid-mediated enhancement of photosynthesis attributes and antioxidant capacity contributes to yield improvement of maize plants under salt stress. J. Plant Grow. Regul. 2018, 37, 1318–1330.
- Yin, Q.-S.; Yuan, X.; Jiang, Y.-G.; Huang, L.-L.; Li, G.-Z.; Hao, L. Salicylic acid-mediated alleviation in NO2 phytotoxicity correlated to increased expression levels of the genes related to photosynthesis and carbon metabolism in Arabidopsis. Environ. Exp. Bot. 2018, 156, 141–150.
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Poll. Res. 2015, 22, 8148–8162.
- Pinto, A.; De Varennes, A.; Fonseca, R.; Teixeira, D.M. Phytoremediation of soils contaminated with heavy metals: Techniques and strategies. In Phytoremediation; Springer: Dordrecht, The Netherlands, 2015; pp. 133–155.
- Kumar, A.; Usmani, Z.; Ahirwal, J.; Rani, P. Phytomanagement of chromium contaminated brown fields. In Phytomanagement of Polluted Sites; Elsevier: Amsterdam, The Neitherland, 2019; pp. 447–469.
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.-W. Role of organic amendments on enhanced bioremediation of heavy metal (loid) contaminated soils. J. Haz. Mat. 2011, 185, 549–574.
- Kumar, V.; Sharma, A.; Kaur, P.; Sidhu, G.P.S.; Bali, A.S.; Bhardwaj, R.; Thukral, A.K.; Cerda, A. Pollution assessment of heavy metals in soils of India and ecological risk assessment: A state-of-the-art. Chemosphere 2019, 216, 449–462.
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Effect of lead on oxidative status, antioxidative response and metal accumulation in Coronopus didymus. Plant Physiol. Biochem. 2016, 105, 290–296.
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Tolerance and hyperaccumulation of cadmium by a wild, unpalatable herb Coronopus didymus (L.) Sm.(Brassicaceae). Ecotox. Environ. Safety 2017, 135, 209–215.
- Sidhu, G.P.S.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Appraising the role of environment friendly chelants in alleviating lead by Coronopus didymus from Pb-contaminated soils. Chemosphere 2017, 182, 129–136.
- Keesstra, S.; Mol, G.; de Leeuw, J.; Okx, J.; de Cleen, M.; Visser, S. Soil-related sustainable development goals: Four concepts to make land degradation neutrality and restoration work. Land 2018, 7, 133.
- Ramzani, P.M.A.; Iqbal, M.; Kausar, S.; Ali, S.; Rizwan, M.; Virk, Z.A. Effect of different amendments on rice (Oryza sativa L.) growth, yield, nutrient uptake and grain quality in Ni-contaminated soil. Environ. Sci. Poll. Res. 2016, 23, 18585–18595.
- Ihedioha, J.; Ukoha, P.; Ekere, N. Ecological and human health risk assessment of heavy metal contamination in soil of a municipal solid waste dump in Uyo, Nigeria. Environ. Geochem. Health 2017, 39, 497–515.
- Van Nevel, L.; Mertens, J.; Staelens, J.; de Schrijver, A.; Tack, F.M.; de Neve, S.; Meers, E.; Verheyen, K. Elevated Cd and Zn uptake by aspen limits the phytostabilization potential compared to five other tree species. Ecol. Eng. 2011, 37, 1072–1080.
- Arshad, T.; Maqbool, N.; Javed, F.; Wahid, A.; Arshad, M.U. Enhancing the defensive mechanism of lead affected barley (Hordeum vulgare L.) genotypes by exogenously applied salicylic acid. J. Agric. Sci. 2017, 9, 139–146.
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875.
- Guerra, F.; Gainza, F.; Pérez, R.; Zamudio, F. Phytoremediation of heavy metals using poplars (Populus spp.): A glimpse of the plant responses to copper, cadmium and zinc stress. In Handbook of Phytoremediation; Nova Science: New York, NY, USA, 2011; pp. 387–413.
- Rascio, N.; Dalla Vecchia, F.; La Rocca, N.; Barbato, R.; Pagliano, C.; Raviolo, M.; Gonnelli, C.; Gabbrielli, R. Metal accumulation and damage in rice (cv. Vialone nano) seedlings exposed to cadmium. Environ. Exp. Bot. 2008, 62, 267–278.
- Baryla, A.; Carrier, P.; Franck, F.; Coulomb, C.; Sahut, C.; Havaux, M. Leaf chlorosis in oilseed rape plants (Brassica napus) grown on cadmium-polluted soil: Causes and consequences for photosynthesis and growth. Planta 2001, 212, 696–709.
- Di Cagno, R.; Guidi, L.; De Gara, L.; Soldatini, G. Combined cadmium and ozone treatments affect photosynthesis and ascorbate-dependent defences in sunflower. New Phytol. 2001, 151, 627–636.
- Küpper, H.; Parameswaran, A.; Leitenmaier, B.; Trtílek, M.; Šetlík, I. Cadmium-induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 2007, 175, 655–674.
- TRAN, T.A.; Popova, L.P. Functions and toxicity of cadmium in plants: Recent advances and future prospects. Turkish J. Bot. 2013, 37, 1–13.
- Wahid, A.; Ghani, A.; Javed, F. Effect of cadmium on photosynthesis, nutrition and growth of mungbean. Agr. Sustain. Develop. 2008, 28, 273–280.
- Tandon, P.K.; Srivastava, P. Growth and metabolism of sesame (Sesamum indicum L.) plants in relation to lead toxicity. Agricul. Sci. 2014, 6, 91–92.
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181.
- Kadukova, J.; Kavuličova, J. Phytoremediation of heavy metal contaminated soils—Plant stress assessment. Handbook of Phytoremediation; Nova Science: New York, NY, USA, 2011; pp. 185–222.
- Baxter, A.; Mittler, R.; Suzuki, N. ROS as key players in plant stress signalling. J. Exp. Bot. 2014, 65, 1229–1240.
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19.
- Cotrozzi, L.; Pellegrini, E.; Guidi, L.; Landi, M.; Lorenzini, G.; Massai, R.; Remorini, D.; Tonelli, M.; Trivellini, A.; Vernieri, P.; et al. Losing the warning signal: Drought compromises the cross-talk of signaling molecules in Quercus ilex exposed to ozone. Front. Plant Sci. 2017, 8, 1020.
- Landi, M.; Cotrozzi, L.; Pellegrini, E.; Remorini, D.; Tonelli, M.; Trivellini, A.; Nali, C.; Guidi, L.; Massai, R.; Vernieri, P.; et al. When “thirsty” means “less able to activate the signalling wave trigged by a pulse of ozone”: A case of study in two Mediterranean deciduous oak species with different drought sensitivity. Sci. Total Environ. 2019, 657, 379–390.
- Kaur, G.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Lead (Pb)-induced biochemical and ultrastructural changes in wheat (Triticum aestivum) roots. Protoplasma 2013, 250, 53–62.
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53.
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4.
- Mohsenzadeh, S.; Shahrtash, M.; Mohabatkar, H. Interactive effects of salicylic acid and silicon on some physiological responses of cadmium-stressed maize seedlings. Iranian J. Sci. Tech. (Sciences) 2011, 35, 57–60.
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Ann. Rev. Phytopathol. 2009, 47, 177–206.
- Khan, M.I.R.; Iqbal, N.; Masood, A.; Per, T.S.; Khan, N.A. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation. Plant Sign. Behav. 2013, 8, e26374.
- Hussein, M.; Balbaa, L.; Gaballah, M. Salicylic acid and salinity effects on growth of maize plants. Res. J. Agricul. Biol. Sci. 2007, 3, 321–328.
- Khokon, M.A.R.; Okuma, E.; Hossain, M.A.; Munemasa, S.; Uraji, M.; Nakamura, Y.; Mori, I.C.; Murata, Y. Involvement of extracellular oxidative burst in salicylic acid-induced stomatal closure in Arabidopsis. Plant Cell Environ. 2011, 34, 434–443.
- Larque-Saavedra, A. Stomatal closure in response to acetylsalicylic acid treatment. Zeitschrift für Pflanzenphysiologie 1979, 93, 371–375.
- Larque-Saavedra, A. The antiranspirant effect of acetylsalcylic acid on Phaseolus vulgaris. Physiol. Plant. 1978, 43, 126–128.
- Alaey, M.; Babalar, M.; Naderi, R.; Kafi, M. Effect of pre-and postharvest salicylic acid treatment on physio-chemical attributes in relation to vase-life of rose cut flowers. Postharvest Biol. Technol. 2011, 61, 91–94.
- Divi, U.K.; Rahman, T.; Krishna, P. Brassinosteroid-mediated stress tolerance in Arabidopsis shows interactions with abscisic acid, ethylene and salicylic acid pathways. BMC Plant Biol. 2010, 10, 151.
- Wang, Y.; Hu, J.; Qin, G.; Cui, H.; Wang, Q. Salicylic acid analogues with biological activity may induce chilling tolerance of maize (Zea mays) seeds. Botany 2012, 90, 845–855.
- Zengin, F. Effects of exogenous salicylic acid on growth characteristics and biochemical content of wheat seeds under arsenic stress. J. Environ. Biol. 2015, 36, 249.
- Ghani, A.; Khan, I.; Ahmed, I.; Mustafa, I.; Abd-Ur, R.; Muhammad, N. Amelioration of lead toxicity in Pisum sativum (L.) by foliar application of salicylic acid. J. Environ. Anal. Toxicol 2015, 5, 10–4172.
- Gondor, O.K.; Pál, M.; Darkó, É.; Janda, T.; Szalai, G. Salicylic acid and sodium salicylate alleviate cadmium toxicity to different extents in maize (Zea mays L.). PLoS ONE 2016, 11, e0160157.
- Zhou, Z.S.; Guo, K.; Elbaz, A.A.; Yang, Z.M. Salicylic acid alleviates mercury toxicity by preventing oxidative stress in roots of Medicago sativa. Environ. Exp. Bot. 2009, 65, 27–34.
- Ahmad, P.; Nabi, G.; Ashraf, M. Cadmium-induced oxidative damage in mustard plants can be alleviated by salicylic acid. South Afr. J. Bot. 2011, 77, 36–44.
- Islam, F.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Shahzad, S.M.; Imran, Q.; Ali, I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol. Biochem. 2016, 108, 456–467.
- Song, W.Y.; Yang, H.C.; Shao, H.B.; Zheng, A.Z.; Brestic, M. The alleviative effects of salicylic acid on the activities of catalase and superoxide dismutase in malting barley (Hordeum uhulgare L.) seedling leaves stressed by heavy metals. CLEAN–Soil, Air, Water 2014, 42, 88–97.
- Faraz, A.; Faizan, M.; Sami, F.; Siddiqui, H.; Hayat, S. Supplementation of salicylic acid and citric acid for alleviation of cadmium toxicity to Brassica juncea. J. Plant Growth Regul. 2019, 1–15.
- Hasanuzzaman, M.; Matin, M.A.; Fardus, J.; Hasanuzzaman, M.; Hossain, M.S.; Parvin, K. Foliar application of salicylic acid improves growth and yield attributes by upregulating the antioxidant defense system in Brassica campestris plants grown in lead-amended soils. Acta Agrobot. 2019, 72.
- Lu, Q.; Zhang, T.; Zhang, W.; Su, C.; Yang, Y.; Hu, D.; Xu, Q. Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotox. Environ. Saf. 2018, 147, 500–508.
- Gu, C.-S.; Yang, Y.-H.; Shao, Y.-F.; Wu, K.-W.; Liu, Z.-L. The effects of exogenous salicylic acid on alleviating cadmium toxicity in Nymphaea tetragona Georgi. S. Afr. J. Bot. 2018, 114, 267–271.
- Li, Q.; Wang, G.; Wang, Y.; Yang, D.; Guan, C.; Ji, J. Foliar application of salicylic acid alleviate the cadmium toxicity by modulation the reactive oxygen species in potato. Ecotox. Environ. Saf. 2019, 172, 317–325.
- Tajti, J.; Németh, E.; Glatz, G.; Janda, T.; Pál, M. Pattern of changes in salicylic acid-induced protein kinase (SIPK) gene expression and salicylic acid accumulation in wheat under cadmium exposure. Plant Biol. 2019, 21, 1176–1180.
- Ahmad, B.; Jaleel, H.; Sadiq, Y.; Khan, M.M.A.; Shabbir, A. Response of exogenous salicylic acid on cadmium induced photosynthetic damage, antioxidant metabolism and essential oil production in peppermint. Plant Growth Regul. 2018, 86, 273–286.
- Zanganeh, R.; Jamei, R.; Rahmani, F. Role of salicylic acid and hydrogen sulfide in promoting lead stress tolerance and regulating free amino acid composition in Zea mays L. Acta Physiol. Plant. 2019, 41, 94.
- Alamri, S.A.D.; Siddiqui, M.H.; Al-Khaishany, M.Y.; Ali, H.M.; Al-Amri, A.; AlRabiah, H.K. Exogenous application of salicylic acid improves tolerance of wheat plants to lead stress. Adv. Agric. Sci. 2018, 6, 25–35.
- Zanganeh, R.; Jamei, R.; Rahmani, F. Impacts of seed priming with salicylic acid and sodium hydrosulfide on possible metabolic pathway of two amino acids in maize plant under lead stress. Mol. Biol. Res. Commun. 2018, 7, 83.
- Mabrouk, B.; Kâab, S.; Rezgui, M.; Majdoub, N.; da Silva, J.T.; Kâab, L. Salicylic acid alleviates arsenic and zinc toxicity in the process of reserve mobilization in germinating fenugreek (Trigonella foenum-graecum L.) seeds. S. Afr. J. Bot. 2019, 124, 235–243.
- Kumari, A.; Pandey, N.; Pandey-Rai, S. Exogenous salicylic acid-mediated modulation of arsenic stress tolerance with enhanced accumulation of secondary metabolites and improved size of glandular trichomes in Artemisia annua L. Protoplasma 2018, 255, 139–152.
- Kumari, A.; Pandey-Rai, S. Enhanced arsenic tolerance and secondary metabolism by modulation of gene expression and proteome profile in Artemisia annua L. after application of exogenous salicylic acid. Plant Physiol. Biochem. 2018, 132, 590–602.
- Saidi, I.; Yousfi, N.; Borgi, M.A. Salicylic acid improves the antioxidant ability against arsenic-induced oxidative stress in sunflower (Helianthus annuus) seedling. J. Plant Nutr. 2017, 40, 2326–2335.
- Singh, A.P.; Dixit, G.; Kumar, A.; Mishra, S.; Kumar, N.; Dixit, S.; Singh, P.K.; Dwivedi, S.; Trivedi, P.K.; Pandey, V. A protective role for nitric oxide and salicylic acid for arsenite phytotoxicity in rice (Oryza sativa L.). Plant Physiol. Biochem. 2017, 115, 163–173.
- Sihag, S.; Brar, B.; Joshi, U. Salicylic acid induces amelioration of chromium toxicity and affects antioxidant enzyme activity in Sorghum bicolor L. Int. J. Phytorem. 2019, 21, 293–304.
- Gill, R.A.; Zhang, N.; Ali, B.; Farooq, M.A.; Xu, J.; Gill, M.B.; Mao, B.; Zhou, W. Role of exogenous salicylic acid in regulating physio-morphic and molecular changes under chromium toxicity in black-and yellow-seeded Brassica napus L. Environ. Sci. Poll. Res. 2016, 23, 20483–20496.
- Huda, A.N.; Swaraz, A.; Reza, M.A.; Haque, M.A.; Kabir, A.H. Remediation of chromium toxicity through exogenous salicylic acid in rice (Oryza sativa L.). Water Air Soil Poll. 2016, 227, 278.
- Zaid, A.; Mohammad, F.; Wani, S.H.; Siddique, K.M. Salicylic acid enhances nickel stress tolerance by up-regulating antioxidant defense and glyoxalase systems in mustard plants. Ecotoxicol. Environ. Saf. 2019, 180, 575–587.
- Kotapati, K.V.; Palaka, B.K.; Ampasala, D.R. Alleviation of nickel toxicity in finger millet (Eleusine coracana L.) germinating seedlings by exogenous application of salicylic acid and nitric oxide. Crop J. 2017, 5, 240–250.
- Soltani Maivan, E.; Radjabian, T.; Abrishamchi, P.; Talei, D. Physiological and biochemical responses of Melissa officinalis L. to nickel stress and the protective role of salicylic acid. Arch. Agron. Soil Sci. 2017, 63, 330–343.
- Karimi, N.; Ghasempour, H.-R. Salicylic acid and jasmonic acid restrains nickel toxicity by ameliorating antioxidant defense system in shoots of metallicolous and non-metallicolous Alyssum inflatum Náyr. Populations. Plant Physiol. Biochem. 2019, 135, 450–459.
- Mei, L.; Daud, M.; Ullah, N.; Ali, S.; Khan, M.; Malik, Z.; Zhu, S. Pretreatment with salicylic acid and ascorbic acid significantly mitigate oxidative stress induced by copper in cotton genotypes. Environ. Sci. Poll. Res. 2015, 22, 9922–9931.
- Moravcová, Š.; Tůma, J.; Dučaiová, Z.K.; Waligórski, P.; Kula, M.; Saja, D.; Słomka, A.; Bąba, W.; Libik-Konieczny, M. Influence of salicylic acid pretreatment on seeds germination and some defence mechanisms of Zea mays plants under copper stress. Plant Physiol. Biochem. 2018, 122, 19–30.
