Antibiotics constitute one of the emerging categories of persistent organic pollutants, characterised by their expansion of resistant pathogens. Antibiotic pollutants create a major public health challenge, with already identifiable detrimental effects on human and animal health. A fundamental aspect of controlling and preventing the spread of pollutants is the continuous screening and monitoring of environmental samples. Molecular imprinting is a state-of-the-art technique for designing robust biomimetic receptors called molecularly imprinted polymers (MIPs), which mimic natural biomolecules in target-selective recognition. When integrated with an appropriate sensor transducer, MIP demonstrates a potential for the needed environmental monitoring, thus justifying the observed rise in interest in this field of research.
- antibiotics
- molecularly imprinted polymers
- Computational Chemistry
- Rational monomer selection
- Challenges for commercial application of MIP
1. Rational Design of Antibiotic Molecularly IPmprinted Polymers (MIPs)
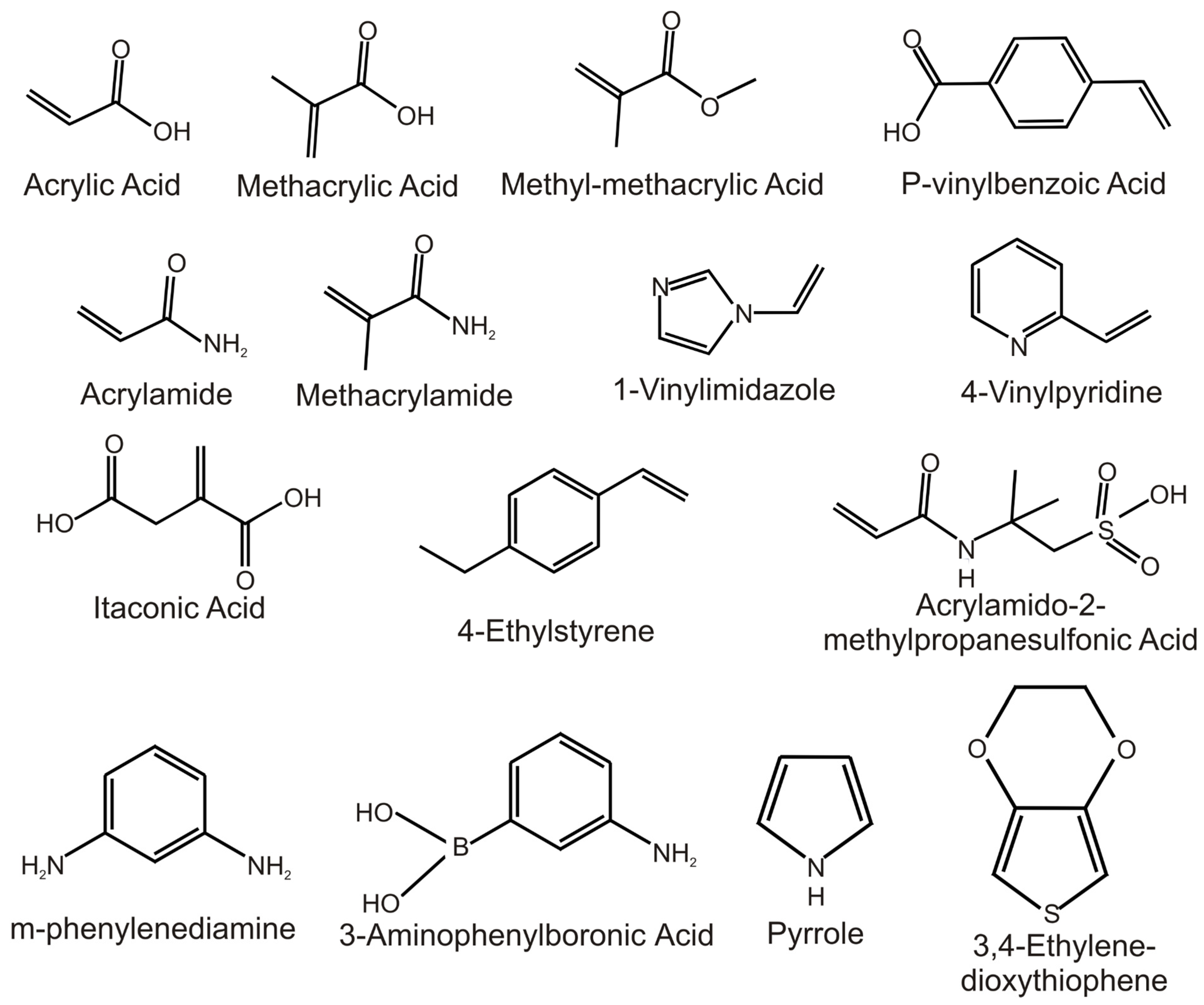
| Computational Approach | Template | Monomers Studied | Binding Energy (kJ/mol) |
Selected Monomer | KD (μM) | QMAX(MIP)/QMAX(NIP) | LOD (nM) | Media | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| QCC | Sulfanilamide | Pyrrole | 18.86 | Pyrrole | - | 20 | Ground water | [67][15] | |
| Furan | 15.11 | ||||||||
| Thiophene | 8.62 | ||||||||
| 1-Methylthiophene | 7.63 | ||||||||
| Methylpyrrole | 7.13 | ||||||||
| Flumequine | Pyrrole | 34.98 | Pyrrole | 20 | - | 1000 | Aquaculture water | [73][21] | |
| Sulfadiazine and sulfamerazine |
AA | 557.727 552.58 |
AA | 0.19 | >3.0 | water | [74][22] | ||
| Sulfamethizole | mPD Pyrrole EDOT |
181.20 80.26 30.10 |
mPD | 47.2 | 8.24 | 2 | Tap water | [75][23] | |
| Amoxicillin | mPD EDOT Pyrrole |
273.05 8.40 63.01 |
mPD | 28.8 | 7.2 | 0.2 | PBS | [68][16] | |
| Azithromycin | 4-ABA Phenol Pyrrole Aniline Thiophene |
71.55 69.41 68.93 45.08 31.96 |
4-ABA | - | - | 80 | River water | [76][24] | |
| Molecular Docking (CDOCKER) |
Norfloxacin | AM AA MAM MAA N-iAA PVP |
82.22 54.27 58.95 100.33 61.04 76.32 |
MAA | 0.004 | 4.3 | 31 | Waste water | [72][20] |
| Norfloxacin | MAA AA MAM AM 4-VP |
87.45 64.68 53.97 76.65 56.48 |
MAA | 2.06 | 2.44 | 16 | Lake water | [71][19] | |
| Molecular Docking (SYBYL) | Cefquinome sulphate | Pyrrole-2-carboxylic acid Pyrrolidine-2-carbohydrazide 4-ABA oPD 4-ATP |
603.09 485.79 435.47 325.77 271.94 |
4-ABA | - | 3.5 | - | PBS | [77][25] |
| Nafcillin | oPD Proline Aniline Pyrrolidine-2-carbohydrazide 4-Aminobenzoic acid |
163.55 159.41 152.67 149.33 135.23 |
oPD | - | - | 80 | River water | [78][26] | |
| Chloramphenicol | AA, MMA | 75 | AA | - | - | 1.5 | Tap water | [79][27] | |
| Molecular Dynamic | Norfloxacin | MAA/EGDMA ratio is optimised | - | MAA, EGDMA | 374; 279 772; 481 |
High adsorption capacity (29.35 mg/g) | - | Water | [80][28] |
| Sulfamethoxazole | APTES/TEOS ratio optimised | - | APTES, TEOS | - | - | 60 | Lake water | [81][29] | |
| Molecular Mechanic | Penicillin G | CMA and CSEV compatibility | - | MAA, TRIM |
- | 6.03–6.69 | - | Water | [82][30] |
2. Practical Challenges of Antibiotic Molecularly Imprinted Polymer
References
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211.
- Qu, S.; Wang, X.; Tong, C.; Wu, J. Metal Ion Mediated Molecularly Imprinted Polymer for Selective Capturing Antibiotics Containing Beta-Diketone Structure. J. Chromatogr. A 2010, 1217, 8205–8211.
- Matsui, J.; Nicholls, I.A.; Takeuchi, T.; Mosbach, K.; Karube, I. Metal Ion Mediated Recognition in Molecularly Imprinted Polymers. Anal. Chim. Acta 1996, 335, 71–77.
- Lay, S.; Ni, X.; Yu, H.; Shen, S. State-of-the-Art Applications of Cyclodextrins as Functional Monomers in Molecular Imprinting Techniques: A Review. J. Sep. Sci. 2016, 39, 2321–2331.
- Wagner, R.; Wan, W.; Biyikal, M.; Benito-Peña, E.; Moreno-Bondi, M.C.; Lazraq, I.; Rurack, K.; Sellergren, B. Synthesis, Spectroscopic, and Analyte-Responsive Behavior of a Polymerizable Naphthalimide-Based Carboxylate Probe and Molecularly Imprinted Polymers Prepared Thereof. J. Org. Chem. 2013, 78, 1377–1389.
- Wang, L.; Lin, Q.; Zhang, Y.; Liu, Y.; Yasin, A.; Zhang, L. Design and Synthesis of Supramolecular Functional Monomers Bearing Urea and Norbornene Motifs. RSC Adv. 2019, 9, 20058–20064.
- Xu, S.; Chen, L.; Li, J.; Guan, Y.; Lu, H. Novel Hg2+-Imprinted Polymers Based on Thymine–Hg2+–Thymine Interaction for Highly Selective Preconcentration of Hg2+ in Water Samples. J. Hazard. Mater. 2012, 237–238, 347–354.
- Zhang, X.; Shen, F.; Zhang, Z.; Xing, Y.; Ren, X. Synthesis of a Novel Cross-Linker Doubles as a Functional Monomer for Preparing a Water Compatible Molecularly Imprinted Polymer. Anal. Methods 2014, 6, 9483–9489.
- Gao, M.; Gao, Y.; Chen, G.; Huang, X.; Xu, X.; Lv, J.; Wang, J.; Xu, D.; Liu, G. Recent Advances and Future Trends in the Detection of Contaminants by Molecularly Imprinted Polymers in Food Samples. Front. Chem. 2020, 8, 616326.
- Muhammad, T.; Nur, Z.; Piletska, E.V.; Yimit, O.; Piletsky, S.A. Rational Design of Molecularly Imprinted Polymer: The Choice of Cross-Linker. Analyst 2012, 137, 2623–2628.
- Zhang, Y.; Song, D.; Lanni, L.M.; Shimizu, K.D. Importance of Functional Monomer Dimerization in the Molecular Imprinting Process. Macromolecules 2010, 43, 6284–6294.
- Azimi, A.; Javanbakht, M. Computational Prediction and Experimental Selectivity Coefficients for Hydroxyzine and Cetirizine Molecularly Imprinted Polymer Based Potentiometric Sensors. Anal. Chim. Acta 2014, 812, 184–190.
- Dong, C.; Li, X.; Guo, Z.; Qi, J. Development of a Model for the Rational Design of Molecular Imprinted Polymer: Computational Approach for Combined Molecular Dynamics/Quantum Mechanics Calculations. Anal. Chim. Acta 2009, 647, 117–124.
- Grabowski, S.J. Chapter 1 Hydrogen Bond—Definitions, Criteria of Existence and Various Types. In Understanding Hydrogen Bonds: Theoretical and Experimental Views; The Royal Society of Chemistry: London, UK, 2021; pp. 1–40. ISBN 978-1-78801-479-3.
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical Detection of Sulfanilamide Using Pencil Graphite Electrode Based on Molecular Imprinting Technology. Electroanalysis 2014, 26, 2328–2336.
- Ayankojo, A.G.; Reut, J.; Boroznjak, R.; Öpik, A.; Syritski, V. Molecularly Imprinted Poly(Meta-Phenylenediamine) Based QCM Sensor for Detecting Amoxicillin. Sens. Actuators B-Chem. 2018, 258, 766–774.
- Xi, S.; Zhang, K.; Xiao, D.; He, H. Computational-Aided Design of Magnetic Ultra-Thin Dummy Molecularly Imprinted Polymer for Selective Extraction and Determination of Morphine from Urine by High-Performance Liquid Chromatography. J. Chromatogr. A 2016, 1473, 1–9.
- Zhang, K.; Zou, W.; Zhao, H.; Dramou, P.; Pham-Huy, C.; He, J.; He, H. Adsorption Behavior of a Computer-Aid Designed Magnetic Molecularly Imprinted Polymer via Response Surface Methodology. RSC Adv. 2015, 5, 61161–61169.
- Fizir, M.; Wei, L.; Muchuan, N.; Itatahine, A.; mehdi, Y.A.; He, H.; Dramou, P. QbD Approach by Computer Aided Design and Response Surface Methodology for Molecularly Imprinted Polymer Based on Magnetic Halloysite Nanotubes for Extraction of Norfloxacin from Real Samples. Talanta 2018, 184, 266–276.
- Niu, M.; Sun, C.; Zhang, K.; Li, G.; Meriem, F.; Pham-Huy, C.; Hui, X.; Shi, J.; He, H. A Simple Extraction Method for Norfloxacin from Pharmaceutical Wastewater with a Magnetic Core–Shell Molecularly Imprinted Polymer with the Aid of Computer Simulation. New J. Chem. 2017, 41, 2614–2624.
- Ktari, N.; Fourati, N.; Zerrouki, C.; Ruan, M.; Seydou, M.; Barbaut, F.; Nal, F.; Yaakoubi, N.; Chehimi, M.M.; Kalfat, R. Design of a Polypyrrole MIP-SAW Sensor for Selective Detection of Flumequine in Aqueous Media. Correlation between Experimental Results and DFT Calculations. RSC Adv. 2015, 5, 88666–88674.
- Chen, L.; Lee, Y.K.; Manmana, Y.; Tay, K.S.; Lee, V.S.; Rahman, N.A. Synthesis, Characterization, and Theoretical Study of an Acrylamide-Based Magnetic Molecularly Imprinted Polymer for the Recognition of Sulfonamide Drugs. E-Polymers 2015, 15, 141–150.
- Ayankojo, A.G.; Tretjakov, A.; Reut, J.; Boroznjak, R.; Öpik, A.; Rappich, J.; Furchner, A.; Hinrichs, K.; Syritski, V. Molecularly Imprinted Polymer Integrated with a Surface Acoustic Wave Technique for Detection of Sulfamethizole. Anal. Chem. 2016, 88, 1476–1484.
- Rebelo, P.; Pacheco, J.G.; Cordeiro, M.N.D.S.; Melo, A.; Delerue-Matos, C. Azithromycin Electrochemical Detection Using a Molecularly Imprinted Polymer Prepared on a Disposable Screen-Printed Electrode. Anal. Methods 2020, 12, 1486–1494.
- Moro, G.; Bottari, F.; Sleegers, N.; Florea, A.; Cowen, T.; Moretto, L.M.; Piletsky, S.; De Wael, K. Conductive Imprinted Polymers for the Direct Electrochemical Detection of β-Lactam Antibiotics: The Case of Cefquinome. Sens. Actuators B Chem. 2019, 297, 126786.
- Bottari, F.; Moro, G.; Sleegers, N.; Florea, A.; Cowen, T.; Piletsky, S.; van Nuijs, A.L.N.; De Wael, K. Electropolymerized O-Phenylenediamine on Graphite Promoting the Electrochemical Detection of Nafcillin. Electroanalysis 2020, 32, 135–141.
- Sai, N.; Wu, Y.; Yu, G.; Sun, Z.; Huang, G. A Novel Enrichment Imprinted Crystalline Colloidal Array for the Ultratrace Detection of Chloramphenicol. Talanta 2016, 161, 1–7.
- Kong, Y.; Wang, N.; Ni, X.; Yu, Q.; Liu, H.; Huang, W.; Xu, W. Molecular Dynamics Simulations of Molecularly Imprinted Polymer Approaches to the Preparation of Selective Materials to Remove Norfloxacin. J. Appl. Polym. Sci. 2016, 133, 42817.
- Xu, W.; Wang, Y.; Huang, W.; Yu, L.; Yang, Y.; Liu, H.; Yang, W. Computer-Aided Design and Synthesis of 2 Core-Shell Molecularly Imprinted Polymers as a Fluorescent Sensor for the Selective Determination of Sulfamethoxazole in Milk and Lake Water. J. Sep. Sci. 2017, 40, 1091–1098.
- Kempe, H.; Kempe, M. QSRR Analysis of β-Lactam Antibiotics on a Penicillin G Targeted MIP Stationary Phase. Anal. Bioanal. Chem. 2010, 398, 3087–3096.
- Ansari, S.; Karimi, M. Recent Progress, Challenges and Trends in Trace Determination of Drug Analysis Using Molecularly Imprinted Solid-Phase Microextraction Technology. Talanta 2017, 164, 612–625.
- Devkota, L.; Nguyen, L.T.; Vu, T.; Piro, B. Electrochemical Determination of Tetracycline Using AuNP-Coated Molecularly Imprinted Overoxidized Polypyrrole Sensing Interface. Electrochim. Acta 2018, 270, 535–542.
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G.; Vasapollo, G.; Del Sole, R.; Mergola, L.; et al. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945.
- Jamieson, O.; Mecozzi, F.; Crapnell, R.D.; Battell, W.; Hudson, A.; Novakovic, K.; Sachdeva, A.; Canfarotta, F.; Herdes, C.; Banks, C.E.; et al. Approaches to the Rational Design of Molecularly Imprinted Polymers Developed for the Selective Extraction or Detection of Antibiotics in Environmental and Food Samples. Phys. Status Solidi A 2021, 218, 2100021.
- BelBruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119.
- Lowdon, J.W.; Diliën, H.; Singla, P.; Peeters, M.; Cleij, T.J.; van Grinsven, B.; Eersels, K. MIPs for Commercial Application in Low-Cost Sensors and Assays—An Overview of the Current Status Quo. Sens. Actuators B Chem. 2020, 325, 128973.
