Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Juhee Ahn and Version 2 by Rita Xu.
With the increasing global threat of antibiotic resistance, there is an urgent need to develop new effective therapies to tackle antibiotic-resistant bacterial infections. Bacteriophage therapy is considered as a possible alternative over antibiotics to treat antibiotic-resistant bacteria. Bacteria can evolve resistance towards bacteriophages through antiphage defense mechanisms, which is a major limitation of phage therapy. The antiphage mechanisms target the phage life cycle, including adsorption, the injection of DNA, synthesis, the assembly of phage particles, and the release of progeny virions.
- phage resistance
- restriction modification
- trade-off
- antibiotic resistance
- CRISPR-Cas
- phage receptor
- anti-phage defence system
- abortive infection
- superinfection exclusion
1. Introduction
Since the discovery of penicillin by Alexander Fleming in 1928, antibiotics have saved millions of lives [1]. However, at the same time, bacteria have evolved antibiotic resistance, exposing the limitation of this magic medicine to treat bacterial infections [2]. The emergence and spread of antibiotic-resistant bacteria have been accelerated due to antibiotic misuse and overuse in medicine and agriculture [3][4][3,4]. A large number of antibiotic-resistant bacteria have been identified as high-priority pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), extended-spectrum β-lactamases (ESBL) producing Enterobacteriaceae, vancomycin-resistant enterococci (VRE), and multidrug-resistant Pseudomonas aeruginosa, Streptococcus pneumonia, Mycobacterium tuberculosis, and Acinetobacter baumannii [5][6][7][8][9][10][5,6,7,8,9,10]. Human health care, animal industries, food manufacturing companies, and agricultural sectors are facing the antibiotic resistance threat [11][12][11,12]. Along with economic losses, antimicrobial resistance tolls 700,000 lives every year throughout the world due to therapeutic failure [13][14][13,14]. Bacteria can rapidly acquire molecular mechanisms to evolve antibiotic resistance through horizontal gene transfer (HGT) from everywhere [15]. The infections caused by multidrug-resistant (MDR) pathogens are difficult to treat because of the limited chemotherapeutic options, which has become a top public health concern worldwide. The frequent antibiotic treatment failures have urged the development of alternative therapeutic agents against MDR pathogens [16].
Bacteriophages (phages) are the most abundant biological entities that specifically infect bacteria [17]. Phages contribute to the diversity of bacterial communities in terms of the coevolutionary fitness dynamics [18][19][18,19]. Recently, phages have gained revived attention as alternative antibacterial agents over conventional antibiotics [20][21][22][23][20,21,22,23]. In Europe, phages have been used for therapeutic and prophylactic purposes, with a less adverse effect on normal microbial flora and no side effects [22][24][22,24]. However, the emergence of phage resistance still remains a major drawback in therapeutic applications [25][26][27][25,26,27]. The emergence of phage resistance is a major concern for the use of phage therapy in terms of the coevolutionary arms races between phages and bacteria [28][29][28,29]. The competitive interactions between phages and bacteria are evolutionary processes, ongoing defense strategies and counterstrategies, for survival in nature [20]. Bacteria evolve phage resistance under selection pressure, resulting in the coincidental changes in bacterial fitness and virulence [4][30][4,30].
2. Phage Structure and Life Cycle
Phages are estimated to outnumber bacteria by tenfold [31]. Phages were discovered in 1917 and first used as therapeutic agents in 1919 [32][33][32,33]. However, the interest in phage therapy was shifted to antibiotics commonly used to treat bacterial infections from the 1940s onwards. Many phages consist of a head/protein capsid containing the genomic material and tail fibers acting as receptor-binding sites [34]. Most phages have polyhedral capsids, predominantly icosahedral, except for filamentous ones [35]. The structure of tailed phages consists of an icosahedral head and a tail with receptor-binding proteins (RBPs) such as tail spikes and tail fibers at the distal end [36][37][36,37]. Phage genomes containing single-stranded or double-stranded RNA or DNA are encased in the protein capsid. RBPs are observed in Myoviridae as long and short fibers attached to the contractile tail, in Podoviridae as spikes or fibers attached to a short non-contractile tail, and in Siphoviridae as baseplates, fibers, spikes, or single straight filaments attached to a long non-contractile tail [38]. The key component of the tail is the contractile sheath. In T4 phages, the contractile sheath contracts to less than half of its original length during the infection to insert the tail tube through the outer membrane for genome delivery [39]. Phages are completely dependent on their hosts for multiplication. Phages have two distinctive life cycles, lytic and lysogenic. In the lytic life cycle, the phages infect the host, multiply inside, and lyse the host cell to release the mature progeny phages. In the lysogenic life cycle, on the contrary, phages integrate their genome into the host’s chromosome, and integrated phage DNA is replicated concurrently with bacterial DNA. In addition, certain temperate phages are maintained as episomal components rather than being incorporated into the host chromosome. The phage DNA integrated into the host cell is termed as the prophage. However, stresses such as UV radiation, antibiotics, pH, temperature, and water activity can activate prophages [32][40][32,40]. When prophage activation is triggered, an irreversible transformation from lysogeny to the lytic life cycle takes place and the phage completes the lytic cycle by making copies and lyses the cell to burst them out [41]. The phage attachment to the bacterial host commences with the binding of RBP on the tip of the phage tail to a target receptor on the host cell surface, occurring in three steps—initial contact, reversible adherence and irreversible attachment [42][43][42,43]. The initial step is the random collision of the phage with the hosts and the recognition of the receptors on the host surface [42]. After phage receptor recognition, the phage RBP reversibly binds with the receptor [44]. The last step is the irreversible phage binding to the receptor [45]. After the permanent binding with the receptor, the phage ejects the genetic material into the host cytoplasm [46] and the phage genome is expressed to produce virion particles such as head, tail, base plate, and fiber, followed by simultaneous assembly. After the phage assembly, phage-encoding enzymes such as endolysin and holin help release the progenies from the host cells [47].3. Coevolutionary Dynamics of Phage-Bacteria Interactions
Bacteria evolve phage resistance through bacteria defense systems under phage selection pressure [48][49][48,49] (Figure 1). In this context, phages, however, evolve counter adaptations against bacterial antiphage mechanisms [27][50][27,50]. Therefore, phages and bacteria can undergo continuous coevolutionary processes involving phage infection and antiphage defense mechanisms [21][27][50][51][52][21,27,50,51,52]. The phage resistance mechanisms of bacteria include non-specific adaptations and specific adaptation systems [48][49][48,49]. The non-specific bacterial defense mechanisms (innate immune systems) against phages include the inhibition of phage attachment to the host surface receptors, the prevention of phage genome entry into the host cells, the restriction of secondary phage infection (superinfection exclusion), the activation of endonucleases and methyltransferases (restriction-modification system), and the induction of suicide in infected cell (abortive infection system) [48]. The phage-specific bacterial defense mechanisms (adaptive immune systems) are a second line of antiphage defense systems such as clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated (Cas) proteins [53]. The phage-resistant bacteria result in phenotypic and genotypic changes, including growth rate, membrane permeability, capsular polysaccharide (CPS) production, phage-binding receptor, virulence, and antibiotic susceptibility [54][55][54,55]. The antiphage mechanisms in bacteria developed against phage infection stages, including adsorption, penetration, synthesis, assembly, and release [56][57][58][59][56,57,58,59].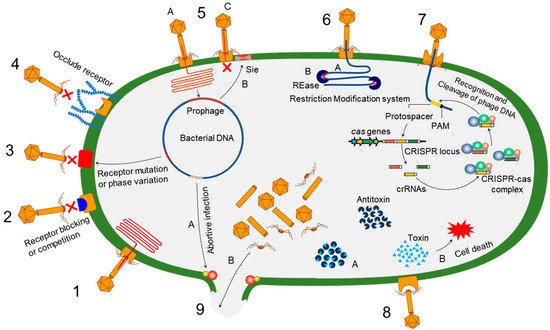
Figure 1. Overview of bacterial antiphage defense mechanisms. 1. Phage infection: successful phage attachment and infection; 2. competition in receptor binding: blocking of the phage-binding receptor to evade phage attachment; 3. alteration in cell surface receptor: mutation or phase variation in the receptor to avoid phage attachment; 4. hiding phage receptor: production of extracellular polysaccharides to hide phage receptor; 5. superinfection exclusion—A: integration of prophage into host genome; B: expression of protein to block DNA entry; C: exclusion of superinfection; 6. restriction-modification system—A: recognition of restriction site; B: cleaving of inserted phage DNA; 7. CRISPR–Cas immunity; 8. toxin–antitoxin immunity—A: antitoxin neutralizes toxin before phage infection; B: phage infection-mediated liberation of toxin to induce reduced metabolism or cell death; 9. abortive infection—A: phage infection mediates expression of abortive infection mechanism; B: release of unassembled phage particles from the host cell.
