Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Dongxu Zhu and Version 2 by Sirius Huang.
One of the environmental problems causing concern in the world today is the black pollution caused by the accumulation of waste rubber resources. Rubber concrete can help solve the black pollution problem caused by waste rubber tires, but it is undeniable that rubber particles will reduce the mechanical properties of concrete. Research on waste rubber particles, particle size and properties are needed for the modification of rubberized concrete.
- rubber
- composition
- chemical components
1. Type and Size of Rubber Particles
At present, there are as many as 25 kinds of rubber available on the market [1]. These kinds have different practical application fields. Automobile tires mainly use natural rubber, or natural rubber and carbon black composite materials, so the waste tire rubber involved in rubber concrete research is generally natural rubber or more complex synthetic rubber material. The utilization of waste tire rubber produced every year is shown in Table 1. Table 1 shows that 30% of the rubber is directly crushed and then landfilled. This treatment method not only wastes rubber resources, but also pollutes the environment.
found that rubber particles often play an unfavorable role in the failure process of rubberized concrete. He believes that this is due to the soft and elastic properties of rubber. The larger the size of the rubber particles, the more space they occupy in the rubber concrete, and the more severe the failure response of the structure under load [5][23].
2. Properties of Rubber Particles
When conducting the test of rubber concrete, scholars choose different types and sizes of rubber particles, which are summarized in Table 2 for the convenience of readers.
Table 2.
Rubber particle size statistics table.
| Reference | CR Size | Reference | CR Size | Reference | CR Size |
|---|---|---|---|---|---|
| Steyn et al. [6] | |||||
| Reutilization | 5–23% | ||||
| Recovery (energy) | 25–60% | ||||
| Stacking | 20–30% |
Forrest [2][3] pointed out that rubber can be recycled in many ways, but it cannot be widely used due to the high energy consumption caused by technology shortages at present. Forrest thinks it is possible to simply reprocess rubber for reuse, such as turning it into coarse particles that can be used as aggregate in concrete [3][2]. Roychand [4][22]
| [ | |||||
| 24] | <4.75 mm (fine) | Taak et al. [7][25] | 10–20 mm (coarse) | Dehdezi et al. [8][26] | 2–4 mm (fine) |
| Tiwari et al. [9][27] | <4.75 mm (fine) | Mhaya et al. [10][9] | 1–4 mm (fine), 5–8 mm (coarse) | Abdelmonem et al. [11][28] | 0–4 mm (fine) |
| Ramdani et al. [12][29] | 0.2–4 mm (fine) | Karunarathna et al. [5][23] | 2–4 mm (fine), 15 mm(coarse) | Othman et al. [13][30] | 180 µm (fine) |
| Ossola. [14][31] | 420–840 µm (fine) | Rahim et al. [ |
Delilah [32][49] pointed out that these impurities include acidic substances and carboxyl groups, which are derived from various admixtures added during the preparation of rubber tires. Rubber tires go through an intensive refining process during the production process, in which oils, additives, accelerators, carbon black and other substances are added. The composition of rubber tires is shown in Table 3, and the substances added during this process cannot be removed by simple mechanical crushing [4][22], as shown in Figure 3. Xiao et al. [35][52] found that low temperature plasma treatment roughened the surface of rubber particles and greatly reduced the water contact angle of rubber. The rough surface of the rubber was able to bond with the cement matrix better.
| Material | Main Ingredients | Composition | ||||||
|---|---|---|---|---|---|---|---|---|
| Rubber | Natural rubber, synthetic rubber | 51% | ||||||
| Reinforcing agent | Carbon black, silica | 25% | ||||||
| Softener | Petroleum process oil, petroleum synthetic resin, etc. | 19.5% | ||||||
| 15 | ] | [32] | 2–4 mm (fine) | Mousavimehr et al. [16][33] | 0–4.75 mm (fine) | |||
| Vulcanizing agent | Sulphur, organic vulcanizers | 1.0% | Barrera et al. [17][34] | 0.85–2.8 mm (fine) | Habib et al. [18][35] | 0.075–10 mm (fine) | Liu et al. [19][36] | <0.42 mm (fine) |
| Vulcanizing accelerator | Thiazole accelerators, sulfenic amide accelerator | 1.5% | Su et al. [20][37] | 0.3 mm, 0.5 mm, 3 mm (fine) | Chaikaew et al. [21][38] | 3.36 mm (fine) | Eisa et al. [22][39] | 2–3 mm (fine) |
| 1–2 mm (fine) | ||||||||
| Vulcanizing accelerator aid | Zinc oxide, stearic acid | 0.5% | Zhu et al. [23][40] | Aslani et al. [24][41] | 2–5 mm (fine) | Shahjalal et al. [25][42] | 4.75–19 mm (fine\coarse) | |
| Antioxidant | Amine antioxidants, phenol antioxidants, wax | 1.5% | Li et al. [26][43] | 0–4 mm (fine) | Wang et al. [27][44] | 1.19–19 mm (fine\coarse) | Gerges et al. [28][45] | 710 µm (fine) |
| Aureliano et al. [29][46] | 0–1.18 mm (fine) | He et al. [30][47] | <0.088 mm (fine) | Liu et al. [31][48] | 0.178, 1.11, 2 mm (fine) |
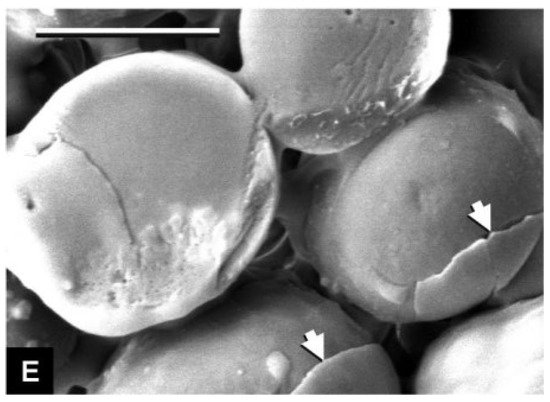
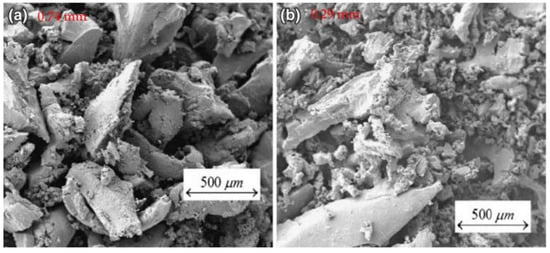
The smoothness of rubber prevents it from bonding effectively to cementitious substrates. Delilah [32][49] put rubber particles into a 0.1 m sodium carbonate buffer containing 3% glutaraldehyde and 2% formaldehyde for special treatment, and the surface of the rubber particles after the removal of impurities was smooth, as shown in Figure 1. In fact, if no special treatment is performed, but only after pulverization, the surface of the rubber particles will appear to be not smooth [33][50], as shown in Figure 2. The impurities remaining on the surface of the rubber particles after crushing will fall off when combined with the cement matrix, and there is no tight bond between the rubber particles and the impurities. Deliah’s research revealed the true appearance of rubber particles, and Segre [34][51] also observed the same phenomenon.
| Filler |
| Calcium carbonate, clay |
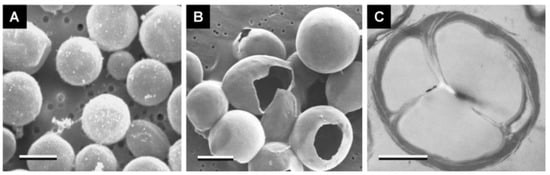
Rubber is a highly elastic material that can recover when subjected to external forces. The high elastic properties of rubber can make up for the high brittleness of concrete, while the low elastic modulus of concrete has always been the focus of research. The work of Karunarathna [5][23] shows that the elastic modulus of concrete is obviously reduced after the incorporation of rubber, and rubber can delay the development of structural cracks. Meanwhile, rubber particles can also act as bridges between cracks when concrete is damaged by force, as shown in Figure 4.
Rubber is a kind of porous material. Karunarathna confirmed the high gas content of rubber through research and photographed overflow bubbles on rubber surfaces when soaked in water, as shown in Figure 5. The porous structure of rubber allows it to contain more air, resulting in weak adhesion between rubber and cement matrix when mixed with concrete. Roychand [4][22] expressed that the problem of high gas content in rubber can be effectively improved by soaking the rubber in advance. Roychand believes that the porous structure in rubber is derived from the linear structure of the internal material composition, which guarantees the high elastic properties of the rubber.

Through Fourier transform infrared and X-ray fluorescence analysis, Jusli et al. [36][53] found that the main chemical components of broken tire rubber particles were carbon, zinc, silicon, magnesium and calcium. The main chemical components are shown in Table 4. According to Table 4, except for SBR, carbon black accounts for the vast majority. In addition, there are admixtures such as oil that cause crushed tire rubber to act differently to rubber alone. This is also a factor to consider when recycling scrap tire rubber. Delilah et al. [32][49] purified natural rubber using lactic acid bacteria and latex and concluded that natural rubber is composed of a rubber core and a protein coating by low-temperature plasma monorail scanning and protein chemical analysis, as shown in Figure 6.