Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Duaa Elhag.
Inflammatory bowel disease (IBD) is a chronic immune-mediated inflammation of the gastrointestinal tract with a highly heterogeneous presentation. It has a relapsing and remitting clinical course that necessitates lifelong monitoring and treatment. Although the availability of a variety of effective therapeutic options including immunomodulators and biologics (such as TNF, CAM inhibitors) has led to a paradigm shift in the treatment outcomes and clinical management of IBD patients, some patients still either fail to respond or lose their responsiveness to therapy over time.
- precision medicine
- Crohn’s disease
- Ulcerative colitis
- Emerging therapies
- IBD activity
1. Introduction
Inflammatory bowel disease (IBD) is a chronic relapsing inflammatory disorder of the gastrointestinal (GI) tract [1]. Multiple factors including urbanization, westernization, dietary changes, increased antimicrobial exposure, and other factors affecting host–microbial homeostasis have been linked to an increase in the prevalence of IBD [2]. IBD is a chronic disease that causes progressive structural and functional damage to the GI tract and intestinal epithelium [3] requiring lifelong medication [1]. IBD is classified into two major subtypes based on pathological features and disease manifestation: Ulcerative Colitis (UC), which primarily affects the colon, and Crohn’s disease (CD), which affects multiple GI sites, suggesting that these subtypes are distinct clinical entities that require distinct clinical management [4][5]. CD and UC are considered highly heterogeneous and complex, which further complicates the clinical management and treatment plans for those patients [5].
A better understanding of disease biology and heterogeneity has resulted in the development of broad-spectrum and disease-specific molecules employed for precise targeting, resulting in a major improvement in therapy effectiveness and outcomes [6]. Though developing treat-to-target techniques has improved IBD patients’ quality of life, wresearchers still face a considerable therapeutic ceiling [7], since a significant proportion of patients either do not react to therapy or lose response over time [8]. Although the mechanisms driving the lower efficacy of IBD medications are unknown, the ability to anticipate treatment response would allow patients with refractory conditions to receive individualized treatment options.
2. Disease Classification, Activity and Severity Assessment Tools
IBD has historically been subclassified into two subtypes CD and UC, though it is a highly heterogeneous condition; therefore, its disease spectrum and complexity cannot be explained by a single CD or UC phenotype. The disease spectrum of IBD is affected by multiple factors such as age of onset of disease, genetic background, microbiome, dietary habits, clinical aspects and disease location classification (for example small bowel-predominant CD is different from colonic predominant CD or left sided UC is different from extensive UC that progressed), disease granularity (rectal involvement or colonic extension) and disease behavior (fibrosing or penetrating) [5]. Besides the disease complexity of IBD subtypes, some other pathologies can also mimic IBD-like disease such as intestinal Behçet, Mediterranean fever enterocolitis, and other microbial infectious causes (including Entamoeba) [5]. The IBD heterogenicity and complexity can significantly influence the treatment outcomes and clinical management of patients. For example, up to 30% of patients do not respond to initial therapy and even among initial responders, 13–46% lose response over time with estimates varying by treatment and disease subtypes [9], a percentage that can sometimes reach as high as 64% after treatment [10]. Therefore, a periodic assessment of IBD activity and disease severity is required to assess disease phenotype, including disease extent and severity in UC, as well as disease extent and disease behavior in CD, to provide a tailored therapy algorithm to every patient [5][11][12][13]. Disease activity in IBD patients is evaluated by combining multiple invasive and/or non-invasive procedures such as patient-reported symptoms, inflammatory markers score, endoscopic assessment, capsule endoscopy, single- or double- balloon enteroscopy, MRI scores, and histology scores [8][14][15][16][17][18][19][20][21][22]. Endoscopic assessment of the gastrointestinal tract is known to be the gold standard method for assessing disease activity, and it has a good correlation with serological markers; however, because endoscopic assessment is an invasive method, it cannot be performed routinely to monitor disease severity [23][24][25][26][27][28][29][30][31]. As a result, non-invasive IBD activity markers, such as fecal markers and serological markers, are advantageous for monitoring disease severity. Table 1 summarizes the various methods used to track disease activity in IBD patients. To grade disease activity, these methods combine patient-reported symptoms (such as the number of stools per day, abdominal pain, and rectal bleeding) with extraintestinal manifestations, physical examination findings, endoscopy results, and hematocrit [32][33][34][35][36][37][38].Table 1. Commonly used IBD activity indices to measure the disease severity.
| CD and IBD-U Activity Indexes | UC Activity Indexes | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Crohn’s Disease Activity index (CDAI) |
| Ulcerative colitis disease activity index (UCDAI) |
| ||||||
| Pediatric Crohn’s Disease Activity index (PCDAI): |
| Pediatric Ulcerative Colitis Activity Index (PUCAI) |
| ||||||
| Weighted Pediatric Crohn’s Disease Activity index (wPCDAI) |
| Ulcerative Colitis Endoscopic Index of Severity (UCEIS) |
| ||||||
| Harvey-Bradshaw index (HBI) or simple endoscopic score |
| Mayo clinic score |
| ||||||
| Mucosal Inflammation Non-invasive index (MINI): |
| Simple Clinical Colitis Activity Index (SCCAI) |
| ||||||
| The simple endoscopic score for CD (SES-CD) |
| The Modified Baron Score |
| ||||||
| The magnetic resonance index of activity (MARIA) and the Clermont score |
| Novel integral disease index of UC activity (NIDI) or Yamamoto-Furusho Index |
| ||||||
| The Lewis score (LS) and Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) |
| UC Colonoscopic Index of Severity (UCC) |
| The Walmsley index |
|
3. Treatment Options for CD and UC
IBD has no known cure. Based on recent treatment strategies, the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE)-II encompasses evidence-based recommendations for IBD patients [39]. The first short-term target of IBD treatment is to control the acute GI inflammation that causes signs and symptoms, which usually results in not only symptom relief but also long-term symptomatic remission and normalizing CRP to reduce further complications. Currently, IBD management has been centered on symptomatic response and endoscopic healing, with four main goals: [1] symptomatic relief, defined as an immediate goal, acknowledging that this is rated highest by patients; [2] symptomatic remission and normalization of CRP, defined as preventing disease flare-ups; [3] decreasing calprotectin and improving the patient’s quality of life and normal growth; and [4] Endoscopic healing with clinical remission in absence of disability. In addition, transmural healing in CD patients and histological healing in UC patients are newly recommended adjunctive measures of the depth of treatment response but are not yet endorsed as formal new treatment targets [39]. Although oral aminosalicylates and corticosteroids are highly effective in suppressing acute GI inflammation, resolving symptoms, and inducing remission, they are unable to reduce long-term complications, improve the patient’s long-term outcomes, or promote healing after mucosal damage. As a result of recent biologic therapy breakthroughs, STRIDE-II encompasses evidence-based recommendations for a paradigm shift in the clinical management of IBD patients, with an emphasis on long-term targets of clinical remission and endoscopic healing in absence of disability, and a restoration of quality of life and normal growth in children [39]. Figure 1 depicts the current STRIDE-II recommendations for therapeutic monitoring of IBD management. The IBD medications fall into the following basic categories: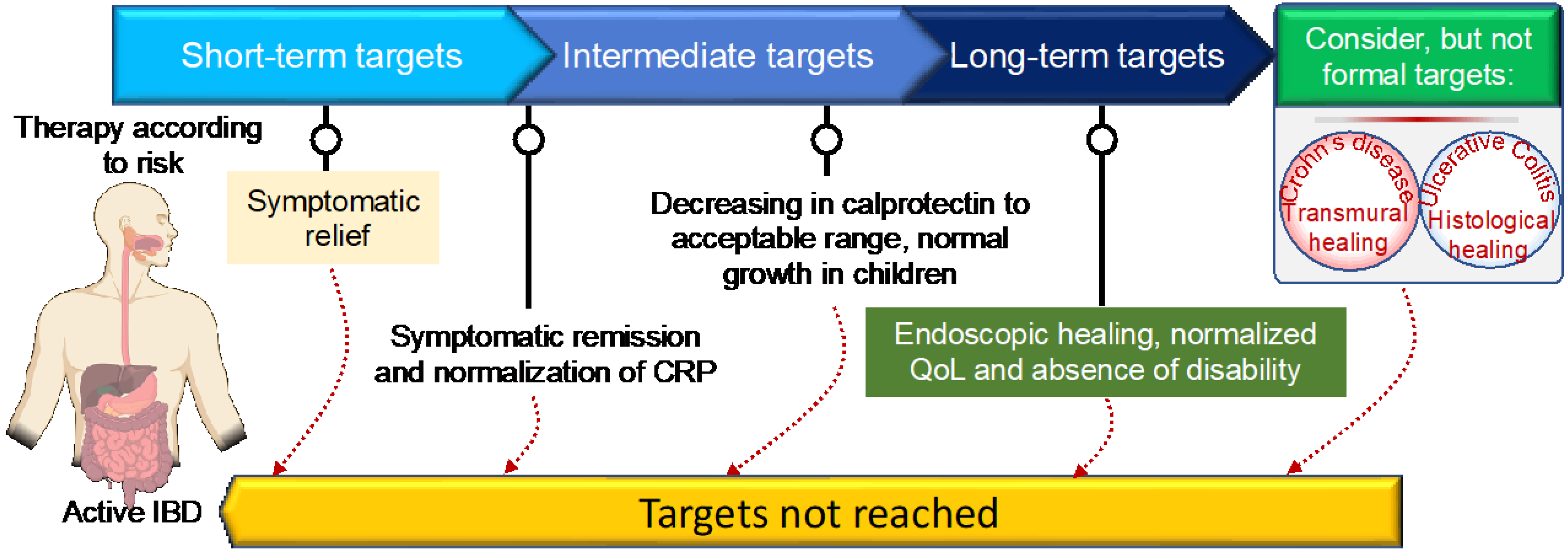
Figure 1. STRIDE-II recommendations for disease monitoring and clinical management of inflammatory bowel disease using short- and long-term target goals.
3.1. Aminosalicylates
These therapies are small molecules that are administered orally or rectally to decrease the inner wall inflammation of the intestines (Figure 2). Aminosalicylates are known to be the first-line treatment option for UC patients with mild-to-moderate disease and the second most prescribed IBD medicine [40][41][42] (Figure 2a,b). Aminosalicylates have a wide range of anti-inflammatory and immunomodulatory functions, including inhibition of cyclooxygenase, lipoxygenase, platelets-activating factor, interleukin (IL)-1 nuclear factor B, and scavenging of reactive oxygen species [43][44][45]. Emerging evidence suggests that aminosalicylates keep IBD patients in remission by preventing leukocyte recruitment into the bowel wall [46][47].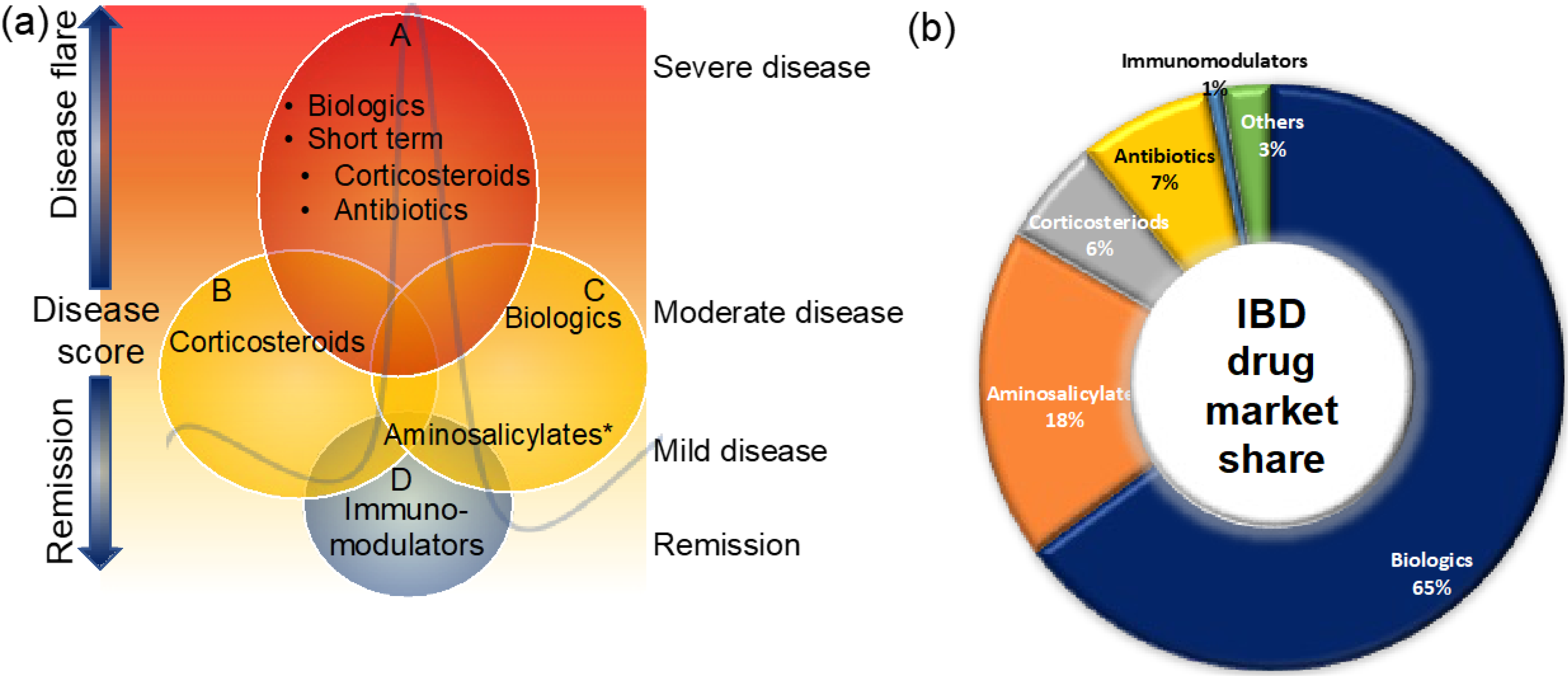
Figure 2. Clinical management of IBD patients during disease flare and remission (a) and the market share of IBD medicines (b). Maintaining remission and prevention of disease flare that triggers signs and symptoms is the main goal of IBD treatment. This figure gives an overview of the current clinical management of IBD patients. For more details, see the main text. * Some aminosalicylates such as balsalazide and mesalamine are approved for mild-to-moderate UC patients.
3.2. Corticosteroids
Corticosteroids are non-selective systemic anti-inflammatory therapies that can be given orally, rectally, or intravenously and are very effective for short-term treatment of moderate-to-severe CD and UC patients [48]. Corticosteroids mediate their immunosuppressive effects by reducing the aberrant production of cytokines such as IL-1, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, TNF-α, IFN-γ, and GM-CSF, according to the mechanism of action studies. [49][50]. The reduced synthesis of proinflammatory cytokines helps in the induction of remission in patients with active IBD. However, their long-term treatment is not recommended due to significant adverse effects such as an increased risk of mortality, infection [51], osteoporosis, psychological disturbances including insomnia, schizophrenia, depression, and euphoria, moon face, fat deposition, dermatological disorders, steroid-induced diabetes [52] and a negative effect on growth in prepubescent children. Given the high clinical demand, many second-generation corticosteroids with improved safety profiles for the clinical management of IBD have emerged in the last two decades (Table 2). Although corticosteroids are very effective at controlling short-term inflammation in IBD patients, they are ineffective at achieving endoscopic remission or healing the mucosa in both UC and CD patients [50][53].Table 2. Therapeutic options for UC and CD.
| Drug Name | Mechanism of Action | Route | Indications | Development Status | |||
|---|---|---|---|---|---|---|---|
| Aminosalicylates |
| * Anti-inflammatory CXY and LXY inhibitor * Anti-inflammatory Prostaglandins inhibitor |
PO PO, rectal PO PO |
Mild-to-mod UC Mild-to-mod UC UC UC |
Approved Approved Approved Approved |
||
| Corticosteroides |
| GRs inhibitor Anti-inflammatory Anti-inflammatory Anti-inflammatory |
PO PO, IV PO PO |
Mild-to-mod CD, UC Mod-to-severe CD, UC Mod-to-severe CD, UC Mod-to-severe CD, UC |
Approved Approved Approved Approved |
||
| Immunomodulators |
| Purine synthesis inhibitor T-cells inhibitor (IL-2) Purine synthesis inhibitor DHFR inhibitor Inhibits IL-2 transcription |
PO PO, IV PO PO, SC PO, IV |
CD, UC UC CD, UC Active CD Mod-to-severe CD, UC |
Approved Approved Approved Approved Approved |
||
| Antibiotics |
| Topo and gyr inhibitor Bacterial DNA synthesis Cell wall synthesis inhibitor Protein synthesis inhibitor Cell wall synthesis inhibitor Bacterial DNA synthesis Protein synthesis inhibitor Cell wall synthesis inhibitor Bacterial DNA synthesis |
PO, IV PO PO PO PO PO |
Active CD and pouchitis Active CD and pouchitis Active CD Active CD Acute severe or chronic UC Acute severe UC |
Approved Approved Approved Approved Approved Approved |
||
| TNF-α inhibitors |
| Anti-TNF-α ab (IgG1) Anti-TNF-α ab Anti-TNF-α ab Anti-TNF-α ab |
SC SC, IV SC SC |
CD, UC Mod-to-severe CD, UC Mod-to-severe CD Mod-to-severe UC (adult) |
Approved Approved Approved Approved |
||
| CAM inhibitors |
| Anti-α4β1-integrin Anti-α4β7-integrin |
IV SC, IV |
Mod-to-severe CD CD, UC |
Approved Approved |
||
| IL-12/-23 inhibitors |
| Anti-IL-12/IL-23 (p40) ab | IV | CD | Approved | ||
| JAK inhibitors |
| Janus Kinase | PO | UC | Approved |
3.3. Immunomodulators
Immunomodulator therapies are administered orally or intravenously to patients to modulate their immune systems and reduce inflammation. Typically, immunomodulators are effective in maintaining remission and are prescribed to patients who are not responding to aminosalicylates and corticosteroids, or as adjuvant treatment to anti-TNF to prevent anti-body formation, particularly with infliximab [54] or as adjuvant treatment to anti-TNF to prevent antibody formation particularly with infliximab [55]. The MOA of different immunomodulators is summarized in Table 2.3.4. Antibiotics
The long-term intestinal inflammation in IBD patients is often associated with gut microbial dysbiosis or intra-abdominal infections [2][56]. In addition, CD is usually associated with abscesses (pockets of pus) or fistulae (connection of diseased bowel to other body part such as bladder, skin, another bowel piece or vagina, which are usually associated with bacterial infections [57]). These microbial infections can mimic the symptoms of an IBD flare. Manipulating the gut microbiota or intestinal infections can be achieved by prebiotics (dietary therapies), fecal transplants (discussed below) and antibiotics. The British Society of Gastroenterology (BSG) recommends the important role of antibiotics for treating secondary complications in CD such as abscesses and bacterial overgrowth [58] and the European Crohn’s and Colitis Organization (ECCO) guidelines recommend the use of antibiotics in case of an acute infection or prior to surgery in UC patients [59]. Therefore, antibiotics are often prescribed for managing IBD patients (including luminal and fistulizing disease for CD and colitis in the case of UC), for treating bacterial infections, or for septic complications of IBD, such as abscesses and post-surgery to prevent disease recurrence [60] (Table 1). Antibiotics may also be used to maintain remissions, or for the treatment of pouchitis [61]. Normally antibiotics are a short-term treatment for IBD patients.3.5. Biologic Therapies
Because many IBD patients do not respond to standard anti-inflammatory and immune modulator medications, there has been a clear need for more specific novel therapeutic approaches to be developed. Bioengineered antibodies that target specific molecules or proteins that cause inflammation or are involved in the inflammatory process are known as biologic therapies [62][63]. Biological therapies are typically prescribed to patients who have moderate-to-severely active disease and have not responded well to conventional therapy [62] (Figure 2). Biologics therapies may be an effective strategy for reducing long-term steroid use as well as maintaining remission; this could be one of the reasons biologics have captured the largest share of the IBD market (Figure 2b). In recent years, there has been a growing trend toward using biologic therapy as first-line therapy in certain clinical situations [64].3.5.1. Specific Treatment Options for CD and UC: Treat-To-Target Approach
Cytokines appear to play a significant role in driving intestinal, systemic, and extra-intestinal inflammation in IBD patients. Targeting pro-inflammatory cytokines such as TNF and other distinct cytokines produced by APCs has already been shown to be effective in suppressing chronic intestinal inflammation, implying that cytokine blockade or targeting cytokine signaling cascades are important fields of interest for clinical management of IBD.3.5.2. TNF-Inhibitors
Given the importance of tumor necrosis factor (TNF) in the pathogenesis of IBD, several TNF-inhibitors have been developed to control intestinal inflammation and the clinical symptoms of IBD (Table 2). TNF-α plays such an important role that anti-TNF agents such as adalimumab, infliximab, certolizumab, and golimumab are now used as standard-of-care therapy for both UC and CD management [65][66]. Interestingly, infliximab has been shown effective in moderate-to-severe UC and CD patients for inducing and maintaining remission, with transmural healing in CD and histological healing in UC, suggesting the broad relevance of anti-TNF-therapy [67]. During intestinal inflammation, TNF is produced by various immune cells including macrophages, T-cells and dendritic cells in the gut of IBD patients [68], to induce neo-angiogenesis [69], activate various mucosal immune cells to produce pro-inflammatory cytokines, and stimulate Paneth cell death via necroptosis [70] or by inducing apoptosis of intestinal epithelial cells [71]. Thus, TNF inhibition can suppress intestinal inflammation through a variety of mechanisms. Recognizing the significant potential of anti-TNF therapies in the treatment of IBD, several biosimilars of TNF-inhibitors have been developed and approved by the Food and Drug Administration (FDA), including adalimumab biosimilars-Hyrimoz™ (adalimumab-adaz), Cyltezo™ (adalimumab-adbm), Amjevita™(adalimumab-atto), infliximab biosimilar-Ixifi™ (infliximab-qbtx), Renflexis™(infliximab-abda), Inflectra™(infliximab-dyyb) [72].3.5.3. CAM Inhibitors
Clinical management of IBD patients has revealed that 30–50 percent of patients either do not respond to anti-TNF therapy or have decreased efficacy over time, implying the need for new alternative therapies [73]. Emerging experimental studies have indicated that inhibitions of activated cell adhesion molecule (CAM) in the inflamed intestinal tissue might provide a new therapeutic option for intestinal inflammation [74]. Natalizumab, the first anti-CAM antibody, was later approved for the treatment of CD patients. Natalizumab has demonstrated significant clinical efficacy in moderate-to-severe CD patients by inhibiting lymphocyte trafficking into the gut via binding to 4-integrins, a ligand known to play an important role in the recruitment of T-cells to intestinal tissues and cause intestinal inflammation [75]. The clinical efficacy was mediated by inhibiting the interaction between α4β7 in the gut and the α4β1 in the blood brain barrier with their ligands (VCAM1 and MAdCAM1, respectively), affecting the homing of immune cells across the gut endothelium and blood–brain barrier, respectively [76][77]. However, despite potent clinical efficacy, long-term natalizumab treatment resulted in a rare but lethal John Cunningham virus (JCV) infection [77][78]. The JCV infection was probably associated with the nonspecific binding mechanism of natalizumab [77][78], highlighting the need for a more specific blockade of α4β7-integrins. Following that, more specific monoclonal IgG antibodies, such as vedolizumab, were developed for moderate-to-severe UC (Table 2), and a few more are currently in clinical trials. Vedolizumab is a novel monoclonal IgG1 antibody that inhibits lymphocyte trafficking into the gut while not interfering with the blood–brain barrier [79][80]. The efficacy of vedolizumab is mediated through the selective blocking of lymphocyte binding to α4β7 integrin in patients with moderate-to-severe IBD [79][80]. The specific inhibition of β7 integrin has been shown to lower the incidence of systemic side effects and to induce long term clinical remission [81][82]. Considering the success of the anti-α4β7 integrin approach, emerging therapies targeting T-cell homing such as etrolizumab, a selective inhibitor of both α4β7 and αEβ7 integrins and ontamalimab, a selective binding inhibitor of MAdCAM-1 to the α4β7 ligand, are the emerging new monoclonal IgG1 and IgG2 antibodies for moderate-to-severe UC and CD patients [79]. AJM300 is another orally active humanized anti-α4 integrin antagonist, inhibits the binding of α4β1 with VCAM-1 and α4β7 with MAdCAM [83] in clinical development for UC patients.3.5.4. Anti-Interleukin Inhibitors
Ustekinumab is a newly approved biologic treatment that targets the p40 subunit of interleukin-12 (IL-12) and IL-23 which are proinflammatory cytokines that play a role in the pathogenesis of IBD [84][85]. It has been approved by FDA for the treatment of adult IBD patients with moderate-to-severe disease. Ustekinumab has shown effectiveness in inducing and maintaining clinical remission in active CD and UC patients [85][86]. Risankizumab is another humanized monoclonal IgG1 antibody that targets the p19 subunit of IL-23 in clinical development. IL-23 is known to play a substantial role in the regulation of the T-helper 17 cells and stimulation of pro-inflammatory cytokines in IBD patients [87]. Preliminary clinical trial results indicate that Risankizumab is well tolerated and able to mediate long-term clinical response and endoscopic remission in active CD patients [88].3.6. JAK Inhibitors
Following the success of biologics in the clinical management of IBD patients, there has been intensive research for alternative effective anti-cytokine strategies. Tofacitinib (CP-690,550) is the first-in-class, oral, pan-Janus kinase (JAK) inhibitor known to be effective and safe for moderate-to-severe UC patients [89] (Table 2). MOA studies reveal that Tofacitinib inhibits JAK-1, JAK-2, and JAK-3 and thereby blocks the signaling pathway of gamma chain-containing cytokines, mainly IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. Interestingly, JAK inhibition has been found to be effective in suppressing T-cells, natural-killer cells, and modulating proinflammatory cytokines; something which has opened the possibility of blocking the activity of several proinflammatory cytokines simultaneously [90]. Indeed, various JAK inhibitors filgotinib (formerly called GLPG0634, GS-6034), PF-06651600, TD-1473, etc., are being evaluated in different clinical trials. Although preliminary clinical results suggest efficacy in moderate-to-severe IBD patients, their safety profiles must be determined in larger phase III clinical trials.3.7. Dietary Therapies
The link between dietary intake and intestinal inflammation has substantially altered ouresearchers preference for dietary changes in the clinical management of IBD [91]. Dietary intake may facilitate intestinal inflammation through various mechanisms including modulating the gut microbiome, tight junctions, and mucous layer [92]. Therefore, various dietary therapies, such as exclusive enteral nutrition (EEN) and CD exclusion diet etc., have been explored in recent years for their potent therapeutic role in the management of IBD patients. EEN is the most widely studied and replicated dietary intervention for CD patients, including pediatric patients, with primary outcomes focusing on induction of clinical remission and mucosal healing [93][94]. Multiple emerging studies indicate that EEN mediates therapeutic effects through modulation of the gut microbiota, by affecting the gut permeability, and by stimulating the immune system, which in-term might lead to endoscopic remission in patients with mild-to-moderate CD [91][95]. Although EEN can help in controlling intestinal inflammation by avoiding the potentially harmful dietary components, the exclusive character of EEN, in which either exclusive or partial formula-based diets are used, is still controversial [96]. Based on the EEN data, more tolerable but still effective solid foods have been explored, such as the new CD exclusion diet (CDED) [97], CD TReatment-with-EATing (CD-TREAT) [98], the specific carbohydrate diet (SCD) [99] and, interestingly, these data revealed the first promising results, emphasizing the role of diet in controlling inflammation in patients with CD by excluding specific food ingredients (94). These dietary interventions incorporate a large amount of high-quality protein, minimize fat content, and incorporate food items rich in complex carbohydrates including natural foods such as chicken, eggs, potatoes, rice, fruits, and vegetables, to assure the patient’s lean mass growth and restoration [100]. Although these dietary-based treatments are more executable compared to EEN, they still need a strict attachment to the protocols, constraining their adherence over time. Recognizing the potential therapeutic role of dietary therapies in IBD, a plethora of new dietary intervention strategies are currently being explored in clinical trials in IBD that may challenge established treatment regimens in future. For examples, two recent CDED clinical trials on pediatric and adult CD patients identified the effectiveness of both CDED and the partial enteral nutrition (PEN) in inducing remission in individuals with mild-to-moderate CD compared to EEN diet (NCT01728870, NCT02231814) [94][97]. The preliminary results from other dietary based treatments including the specific carbohydrate diet (SCD) or Mediterranean diet (MD) revealed significant clinical and mucosal improvements in IBD patients through a promotion of the gut microbiome and metabolomes associated with remission and lowering the levels of fecal calprotectin [97][101][102]. Interestingly, more promising studies are now investigating the role of nutritional interventions in combination with analyses of gut microbiome and metabolome, aiming to restore the healthy gut microbiome balance and providing a new hope for individuals with IBD (NCT04018040, NCT04552158, NCT02858557).References
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217.
- Kumar, M.; Garand, M.; Al Khodor, S. Integrating omics for a better understanding of Inflammatory Bowel Disease: A step towards personalized medicine. J. Transl. Med. 2019, 17, 419.
- Vasant, D.H.; Ford, A.C. Functional gastrointestinal disorders in inflammatory bowel disease: Time for a paradigm shift? World J. Gastroenterol. 2020, 26, 3712–3719.
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122.
- Verstockt, B.; Bressler, B.; Martinez-Lozano, H.; McGovern, D.; Silverberg, M.S. Time to Revisit Disease Classification in Inflammatory Bowel Disease: Is the Current Classification of Inflammatory Bowel Disease Good Enough for Optimal Clinical Management? Gastroenterology 2022, 162, 1370–1382.
- Neurath, M.F. Targeting immune cell circuits and trafficking in inflammatory bowel disease. Nat. Immunol. 2019, 20, 970–979.
- Alsoud, D.; Verstockt, B.; Fiocchi, C.; Vermeire, S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol. Hepatol. 2021, 6, 589–595.
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn’s Colitis 2018, 13, 144K–164K.
- Plichta, D.R.; Graham, D.B.; Subramanian, S.; Xavier, R.J. Therapeutic Opportunities in Inflammatory Bowel Disease: Mechanistic Dissection of Host-Microbiome Relationships. Cell 2019, 178, 1041–1056.
- Roda, G.; Jharap, B.; Neeraj, N.; Colombel, J.F. Loss of Response to Anti-TNFs: Definition, Epidemiology, and Management. Clin. Transl. Gastroenterol. 2016, 7, e135.
- Liverani, E.; Scaioli, E.; Digby, R.J.; Bellanova, M.; Belluzzi, A. How to predict clinical relapse in inflammatory bowel disease patients. World J. Gastroenterol. 2016, 22, 1017–1033.
- Liu, T.; Han, L.; Tilley, M.; Afzelius, L.; Maciejewski, M.; Jelinsky, S.; Tian, C.; McIntyre, M.; Agee, M.; Auton, A.; et al. Distinct clinical phenotypes for Crohn’s disease derived from patient surveys. BMC Gastroenterol. 2021, 21, 160.
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770.
- Williet, N.; Jardin, S.; Roblin, X. The Simplified Magnetic Resonance Index of Activity (MARIA) for Crohn’s Disease Is Strongly Correlated With the MARIA and Clermont Score: An External Validation. Gastroenterology 2020, 158, 282–283.
- Omori, T.; Kambayashi, H.; Murasugi, S.; Ito, A.; Yonezawa, M.; Nakamura, S.; Tokushige, K. Comparison of Lewis Score and Capsule Endoscopy Crohn’s Disease Activity Index in Patients with Crohn’s Disease. Dig. Dis. Sci. 2020, 65, 1180–1188.
- Bots, S.; Nylund, K.; Löwenberg, M.; Gecse, K.; D’Haens, G. Intestinal Ultrasound to Assess Disease Activity in Ulcerative Colitis: Development of a novel UC-Ultrasound Index. J. Crohn’s Colitis 2021, 15, 1264–1271.
- Buisson, A.; Pereira, B.; Goutte, M.; Reymond, M.; Allimant, C.; Obritin-Guilhen, H.; Bommelaer, G.; Hordonneau, C. Magnetic resonance index of activity (MaRIA) and Clermont score are highly and equally effective MRI indices in detecting mucosal healing in Crohn’s disease. Dig. Liver Dis. 2017, 49, 1211–1217.
- Gui, X.; Bazarova, A.; del Amor, R.; Vieth, M.; de Hertogh, G.; Villanacci, V.; Zardo, D.; Parigi, T.L.; Røyset, E.S.; Shivaji, U.N.; et al. PICaSSO Histologic Remission Index (PHRI) in ulcerative colitis: Development of a novel simplified histological score for monitoring mucosal healing and predicting clinical outcomes and its applicability in an artificial intelligence system. Gut 2022, 71, 889–898.
- D’Amico, F.; Chateau, T.; Laurent, V.; Danese, S.; Peyrin-Biroulet, L. Which MRI Score and Technique Should Be Used for Assessing Crohn’s Disease Activity? J. Clin. Med. 2020, 9, 1691.
- Goodsall, T.M.; Nguyen, T.M.; Parker, C.E.; Ma, C.; Andrews, J.M.; Jairath, V.; Bryant, R.V. Systematic Review: Gastrointestinal Ultrasound Scoring Indices for Inflammatory Bowel Disease. J. Crohns Colitis 2021, 15, 125–142.
- Feuerstein, J.D.; Ho, E.Y.; Shmidt, E.; Singh, H.; Falck-Ytter, Y.; Sultan, S.; Terdiman, J.P. AGA Clinical Practice Guidelines on the Medical Management of Moderate to Severe Luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology 2021, 160, 2496–2508.
- Feuerstein, J.D.; Isaacs, K.L.; Schneider, Y.; Siddique, S.M.; Falck-Ytter, Y.; Singh, S.; on behalf of theAGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the Management of Moderate to Severe Ulcerative Colitis. Gastroenterology 2020, 158, 1450–1461.
- Annese, V.; Daperno, M.; Rutter, M.D.; Amiot, A.; Bossuyt, P.; East, J.; Ferrante, M.; Gotz, M.; Katsanos, K.H.; Kiesslich, R.; et al. European evidence based consensus for endoscopy in inflammatory bowel disease. J. Crohns Colitis 2013, 7, 982–1018.
- Carman, N.; Tomalty, D.; Church, P.C.; Mack, D.R.; Benchimol, E.I.; Otley, A.R.; Jacobson, K.; Huynh, H.Q.; De Bruyn, J.C.; El-Matary, W.; et al. Clinical disease activity and endoscopic severity correlate poorly in children newly diagnosed with Crohn’s disease. Gastrointest. Endosc. 2019, 89, 364–372.
- Samuel, S.; Bruining, D.H.; Loftus, E.V., Jr.; Thia, K.T.; Schroeder, K.W.; Tremaine, W.J.; Faubion, W.A.; Kane, S.V.; Pardi, D.S.; de Groen, P.C.; et al. Validation of the ulcerative colitis colonoscopic index of severity and its correlation with disease activity measures. Clin. Gastroenterol. Hepatol. 2013, 11, 49–54.e1.
- Yamamoto-Furusho, J.K.; Bozada-Gutierrez, K.E.; Sanchez-Rodriguez, A.; Bojalil-Romano, F.; Barreto-Zuniga, R.; Martinez-Benitez, B. Validation of a novel integral disease index for evaluating the grade of activity in Mexican patients with ulcerative colitis: A prospective cohort study. Rev. Gastroenterol. Mex. 2019, 84, 317–325.
- Mohammed Vashist, N.; Samaan, M.; Mosli, M.H.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2018, 1, Cd011450.
- Chen, H.; Wu, L.; Wang, M.; Shao, B.; Ye, L.; Zhang, Y.; Cao, Q. Use of the ulcerative colitis endoscopic index of severity and Mayo endoscopic score for predicting the therapeutic effect of mesalazine in patients with ulcerative colitis. Laparosc. Endosc. Robot. Surg. 2021, 4, 33–39.
- Travis, S.P.L.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.-F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542.
- Balint, A.; Farkas, K.; Szepes, Z.; Nagy, F.; Szucs, M.; Tiszlavicz, L.; Bor, R.; Milassin, A.; Rutka, M.; Fabian, A.; et al. How disease extent can be included in the endoscopic activity index of ulcerative colitis: The panMayo score, a promising scoring system. BMC Gastroenterol. 2018, 18, 7.
- Restellini, S.; Chao, C.Y.; Martel, M.; Barkun, A.; Kherad, O.; Seidman, E.; Wild, G.; Bitton, A.; Afif, W.; Bessissow, T.; et al. Clinical Parameters Correlate With Endoscopic Activity of Ulcerative Colitis: A Systematic Review. Clin. Gastroenterol. Hepatol. 2019, 17, 1265–1275.e8.
- Koliani-Pace, J.L.; Siegel, C.A. Beyond disease activity to overall disease severity in inflammatory bowel disease. Lancet Gastroenterol. Hepatol. 2017, 2, 624–626.
- Walmsley, R.S.; Ayres, R.C.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32.
- Rodrigues, B.L.; Mazzaro, M.C.; Nagasako, C.K.; Ayrizono, M.d.L.S.; Fagundes, J.J.; Leal, R.F. Assessment of disease activity in inflammatory bowel diseases: Non-invasive biomarkers and endoscopic scores. World J. Gastrointest. Endosc. 2020, 12, 504–520.
- Pabla, B.S.; Schwartz, D.A. Assessing Severity of Disease in Patients with Ulcerative Colitis. Gastroenterol. Clin. N. Am 2020, 49, 671–688.
- Dulai, P.S.; Singh, S.; Jairath, V.; Ma, C.; Narula, N.; Vande Casteele, N.; Peyrin-Biroulet, L.; Vermeire, S.; D’Haens, G.; Feagan, B.G.; et al. Prevalence of endoscopic improvement and remission according to patient-reported outcomes in ulcerative colitis. Aliment. Pharmacol. Ther. 2020, 51, 435–445.
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355.
- Kerur, B.; Litman, H.J.; Stern, J.B.; Weber, S.; Lightdale, J.R.; Rufo, P.A.; Bousvaros, A. Correlation of endoscopic disease severity with pediatric ulcerative colitis activity index score in children and young adults with ulcerative colitis. World J. Gastroenterol. 2017, 23, 3322–3329.
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583.
- Nagahori, M.; Kochi, S.; Hanai, H.; Yamamoto, T.; Nakamura, S.; Omuro, S.; Watanabe, M.; Hibi, T.; Group, O.S. Real life results in using 5-ASA for maintaining mild to moderate UC patients in Japan, a multi-center study, OPTIMUM Study. BMC Gastroenterol. 2017, 17, 47.
- Louis, E.; Paridaens, K.; Al Awadhi, S.; Begun, J.; Cheon, J.H.; Dignass, A.U.; Magro, F.; Márquez, J.R.; Moschen, A.R.; Narula, N.; et al. Modelling the benefits of an optimised treatment strategy for 5-ASA in mild-to-moderate ulcerative colitis. BMJ Open Gastroenterol. 2022, 9, e000853.
- Burri, E.; Maillard, M.H.; Schoepfer, A.M.; Seibold, F.; Van Assche, G.; Rivière, P.; Laharie, D.; Manz, M. Treatment Algorithm for Mild and Moderate-to-Severe Ulcerative Colitis: An Update. Digestion 2020, 101 (Suppl. S1), 2–15.
- Nikolaus, S.; Folscn, U.; Schreiber, S. Immunopharmacology of 5-aminosalicylic acid and of glucocorticoids in the therapy of inflammatory bowel disease. Hepatogastroenterology 2000, 47, 71–82.
- Weber, C.K.; Liptay, S.; Wirth, T.; Adler, G.; Schmid, R.M. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology 2000, 119, 1209–1218.
- Allgayer, H.; Kruis, W. Aminosalicylates: Potential antineoplastic actions in colon cancer prevention. Scand. J. Gastroenterol. 2002, 37, 125–131.
- Greenfield, S.M.; Punchard, N.A.; Teare, J.P.; Thompson, R.P. Review article: The mode of action of the aminosalicylates in inflammatory bowel disease. Aliment. Pharmacol. Ther. 1993, 7, 369–383.
- Cai, Z.; Wang, S.; Li, J. Treatment of Inflammatory Bowel Disease: A Comprehensive Review. Front. Med. 2021, 8, 2681.
- Waljee, A.K.; Wiitala, W.L.; Govani, S.; Stidham, R.; Saini, S.; Hou, J.; Feagins, L.A.; Khan, N.; Good, C.B.; Vijan, S.; et al. Corticosteroid Use and Complications in a US Inflammatory Bowel Disease Cohort. PLoS ONE 2016, 11, e0158017.
- Ramamoorthy, S.; Cidlowski, J.A. Corticosteroids: Mechanisms of Action in Health and Disease. Rheum. Dis. Clin. N. Am. 2016, 42, 15–31.
- Strehl, C.; Ehlers, L.; Gaber, T.; Buttgereit, F. Glucocorticoids—All-Rounders Tackling the Versatile Players of the Immune System. Front. Immunol. 2019, 10, 1744.
- Dorrington, A.M.; Selinger, C.P.; Parkes, G.C.; Smith, M.; Pollok, R.C.; Raine, T. The Historical Role and Contemporary Use of Corticosteroids in Inflammatory Bowel Disease. J. Crohns Colitis 2020, 14, 1316–1329.
- An, Y.K. Common mistakes with steroids. J. Gastroenterol. Hepatol. 2021, 36 (Suppl. S1), 30–31.
- Ardizzone, S.; Cassinotti, A.; Duca, P.; Mazzali, C.; Penati, C.; Manes, G.; Marmo, R.; Massari, A.; Molteni, P.; Maconi, G.; et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin. Gastroenterol. Hepatol. 2011, 9, 483–489.e3.
- Melmed, G.Y.; Spiegel, B.M.; Bressler, B.; Cheifetz, A.S.; Devlin, S.M.; Harrell, L.E.; Irving, P.M.; Jones, J.; Kaplan, G.G.; Kozuch, P.L.; et al. The Appropriateness of Concomitant Immunomodulators With Anti–Tumor Necrosis Factor Agents for Crohn’s Disease: One Size Does Not Fit All. Clin. Gastroenterol. Hepatol. 2010, 8, 655–659.
- Raine, T.; Kennedy, N.A. Immunomodulator and Biologic Combination Therapy in IBD: The Debate That Just Won’t Go Away? J. Crohn’s Colitis 2020, 14, 1343–1344.
- Azimi, T.; Nasiri, M.J.; Chirani, A.S.; Pouriran, R.; Dabiri, H. The role of bacteria in the inflammatory bowel disease development: A narrative review. Apmis 2018, 126, 275–283.
- Satoh, K.; Okuyama, M.; Furuya, T.; Irie, Y.; Nakae, H. Severe Sepsis Caused by Bacteria That Entered via the Intestinal Tract: A Case of Crohn’s Disease in a Child. Cureus 2020, 12, e9822.
- Mowat, C.; Cole, A.; Windsor, A.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L.; et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571–607.
- Dignass, A.; Lindsay, J.O.; Sturm, A.; Windsor, A.; Colombel, J.F.; Allez, M.; D’Haens, G.; D’Hoore, A.; Mantzaris, G.; Novacek, G.; et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: Current management. J. Crohns Colitis 2012, 6, 991–1030.
- Ledder, O. Antibiotics in inflammatory bowel diseases: Do we know what we’re doing? Transl. Pediatr. 2019, 8, 42–55.
- Rabbenou, W.; Chang, S. Medical treatment of pouchitis: A guide for the clinician. Ther. Adv. Gastroenterol. 2021, 14, 17562848211023376.
- Paramsothy, S.; Rosenstein, A.K.; Mehandru, S.; Colombel, J.F. The current state of the art for biological therapies and new small molecules in inflammatory bowel disease. Mucosal. Immunol. 2018, 11, 1558–1570.
- Banerjee, R.; Ali, R.A.R.; Wei, S.C.; Adsul, S. Biologics for the Management of Inflammatory Bowel Disease: A Review in Tuberculosis-Endemic Countries. Gut Liver 2020, 14, 685–698.
- Siegel, C.A.; Yang, F.; Eslava, S.; Cai, Z. Treatment Pathways Leading to Biologic Therapies for Ulcerative Colitis and Crohn’s Disease in the United States. Clin. Transl. Gastroenterol. 2020, 11, e00128.
- Sandborn, W.J.; Feagan, B.G.; Marano, C.; Zhang, H.; Strauss, R.; Johanns, J.; Adedokun, O.J.; Guzzo, C.; Colombel, J.F.; Reinisch, W.; et al. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014, 146, 96–109.e1.
- Reinisch, W.; Gecse, K.; Halfvarson, J.; Irving, P.M.; Jahnsen, J.; Peyrin-Biroulet, L.; Rogler, G.; Schreiber, S.; Danese, S. Clinical Practice of Adalimumab and Infliximab Biosimilar Treatment in Adult Patients With Crohn’s Disease. Inflamm. Bowel Dis. 2021, 27, 106–122.
- Papamichael, K.; Lin, S.; Moore, M.; Papaioannou, G.; Sattler, L.; Cheifetz, A.S. Infliximab in inflammatory bowel disease. Ther. Adv. Chronic Dis. 2019, 10, 2040622319838443.
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342.
- Rutella, S.; Fiorino, G.; Vetrano, S.; Correale, C.; Spinelli, A.; Pagano, N.; Arena, V.; Maggiano, N.; Repici, A.; Malesci, A.; et al. Infliximab therapy inhibits inflammation-induced angiogenesis in the mucosa of patients with Crohn’s disease. Am. J. Gastroenterol. 2011, 106, 762–770.
- Gunther, C.; Martini, E.; Wittkopf, N.; Amann, K.; Weigmann, B.; Neumann, H.; Waldner, M.J.; Hedrick, S.M.; Tenzer, S.; Neurath, M.F.; et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 2011, 477, 335–339.
- Van den Brande, J.M.; Koehler, T.C.; Zelinkova, Z.; Bennink, R.J.; te Velde, A.A.; ten Cate, F.J.; van Deventer, S.J.; Peppelenbosch, M.P.; Hommes, D.W. Prediction of antitumour necrosis factor clinical efficacy by real-time visualisation of apoptosis in patients with Crohn’s disease. Gut 2007, 56, 509–517.
- Rudrapatna, V.A.; Velayos, F. Biosimilars for the Treatment of Inflammatory Bowel Disease. Pract. Gastroenterol. 2019, 43, 84–91.
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278.
- Picarella, D.; Hurlbut, P.; Rottman, J.; Shi, X.; Butcher, E.; Ringler, D.J. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J. Immunol. 1997, 158, 2099–2106.
- Kurmaeva, E.; Lord, J.D.; Zhang, S.; Bao, J.R.; Kevil, C.G.; Grisham, M.B.; Ostanin, D.V. T cell-associated α4β7 but not α4β1 integrin is required for the induction and perpetuation of chronic colitis. Mucosal. Immunol. 2014, 7, 1354–1365.
- Sandborn, W.J.; Colombel, J.F.; Enns, R.; Feagan, B.G.; Hanauer, S.B.; Lawrance, I.C.; Panaccione, R.; Sanders, M.; Schreiber, S.; Targan, S.; et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2005, 353, 1912–1925.
- Polman, C.H.; O’Connor, P.W.; Havrdova, E.; Hutchinson, M.; Kappos, L.; Miller, D.H.; Phillips, J.T.; Lublin, F.D.; Giovannoni, G.; Wajgt, A.; et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 2006, 354, 899–910.
- Targan, S.R.; Feagan, B.G.; Fedorak, R.N.; Lashner, B.A.; Panaccione, R.; Present, D.H.; Spehlmann, M.E.; Rutgeerts, P.J.; Tulassay, Z.; Volfova, M.; et al. Natalizumab for the treatment of active Crohn’s disease: Results of the ENCORE Trial. Gastroenterology 2007, 132, 1672–1683.
- D’Amico, F.; Danese, S.; Peyrin-Biroulet, L. Vedolizumab and etrolizumab for ulcerative colitis: Twins or simple cousins? Expert Opin. Biol. Ther. 2020, 20, 353–361.
- Rutgeerts, P.J.; Fedorak, R.N.; Hommes, D.W.; Sturm, A.; Baumgart, D.C.; Bressler, B.; Schreiber, S.; Mansfield, J.C.; Williams, M.; Tang, M.; et al. A randomised phase I study of etrolizumab (rhuMAb beta7) in moderate to severe ulcerative colitis. Gut 2013, 62, 1122–1130.
- Yu, Y.; Zhu, J.; Mi, L.Z.; Walz, T.; Sun, H.; Chen, J.; Springer, T.A. Structural specializations of alpha(4)beta(7), an integrin that mediates rolling adhesion. J. Cell Biol. 2012, 196, 131–146.
- Wyant, T.; Yang, L.; Fedyk, E. In vitro assessment of the effects of vedolizumab binding on peripheral blood lymphocytes. MAbs 2013, 5, 842–850.
- Yoshimura, N.; Watanabe, M.; Motoya, S.; Tominaga, K.; Matsuoka, K.; Iwakiri, R.; Watanabe, K.; Hibi, T.; Group, A.J.M.S. Safety and Efficacy of AJM300, an Oral Antagonist of alpha4 Integrin, in Induction Therapy for Patients With Active Ulcerative Colitis. Gastroenterology 2015, 149, 1775–1783.e2.
- Aggeletopoulou, I.; Assimakopoulos, S.F.; Konstantakis, C.; Triantos, C. Interleukin 12/interleukin 23 pathway: Biological basis and therapeutic effect in patients with Crohn’s disease. World J. Gastroenterol. 2018, 24, 4093–4103.
- Harris, K.A.; Horst, S.; Gadani, A.; Nohl, A.; Annis, K.; Duley, C.; Beaulieu, D.; Ghazi, L.; Schwartz, D.A. Patients with Refractory Crohn’s Disease Successfully Treated with Ustekinumab. Inflamm. Bowel Dis. 2016, 22, 397–401.
- Khorrami, S.; Ginard, D.; Marin-Jimenez, I.; Chaparro, M.; Sierra, M.; Aguas, M.; Sicilia, B.; Garcia-Sanchez, V.; Suarez, C.; Villoria, A.; et al. Ustekinumab for the Treatment of Refractory Crohn’s Disease: The Spanish Experience in a Large Multicentre Open-label Cohort. Inflamm. Bowel Dis. 2016, 22, 1662–1669.
- Geremia, A.; Arancibia-Carcamo, C.V.; Fleming, M.P.; Rust, N.; Singh, B.; Mortensen, N.J.; Travis, S.P.; Powrie, F. IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 2011, 208, 1127–1133.
- Feagan, B.G.; Panes, J.; Ferrante, M.; Kaser, A.; D’Haens, G.R.; Sandborn, W.J.; Louis, E.; Neurath, M.F.; Franchimont, D.; Dewit, O.; et al. Risankizumab in patients with moderate to severe Crohn’s disease: An open-label extension study. Lancet Gastroenterol. Hepatol. 2018, 3, 671–680.
- Weisshof, R.; Golan, M.A.; Yvellez, O.V.; Rubin, D.T. The use of tofacitinib in the treatment of inflammatory bowel disease. Immunotherapy 2018, 10, 837–849.
- Sandborn, W.J.; Ghosh, S.; Panes, J.; Vranic, I.; Su, C.; Rousell, S.; Niezychowski, W.; Study, A.I. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N. Engl. J. Med. 2012, 367, 616–624.
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738.
- Sasson, A.N.; Ananthakrishnan, A.N.; Raman, M. Diet in Treatment of Inflammatory Bowel Diseases. Clin. Gastroenterol. Hepatol. 2021, 19, 425–435.e3.
- Miele, E.; Shamir, R.; Aloi, M.; Assa, A.; Braegger, C.; Bronsky, J.; de Ridder, L.; Escher, J.C.; Hojsak, I.; Kolaček, S.; et al. Nutrition in Pediatric Inflammatory Bowel Disease: A Position Paper on Behalf of the Porto Inflammatory Bowel Disease Group of the European Society of Pediatric Gastroenterology, Hepatology and Nutrition. J. Pediatr. Gastroenterol. Nutr. 2018, 66, 687–708.
- Yanai, H.; Levine, A.; Hirsch, A.; Boneh, R.S.; Kopylov, U.; Eran, H.B.; Cohen, N.A.; Ron, Y.; Goren, I.; Leibovitzh, H.; et al. The Crohn’s disease exclusion diet for induction and maintenance of remission in adults with mild-to-moderate Crohn’s disease (CDED-AD): An open-label, pilot, randomised trial. Lancet Gastroenterol. Hepatol. 2022, 7, 49–59.
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.H.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019, 68 (Suppl. S3), s1–s106.
- Pigneur, B.; Ruemmele, F.M. Nutritional interventions for the treatment of IBD: Current evidence and controversies. Ther. Adv. Gastroenterol. 2019, 12, 1756284819890534.
- Levine, A.; Wine, E.; Assa, A.; Sigall Boneh, R.; Shaoul, R.; Kori, M.; Cohen, S.; Peleg, S.; Shamaly, H.; On, A.; et al. Crohn’s Disease Exclusion Diet Plus Partial Enteral Nutrition Induces Sustained Remission in a Randomized Controlled Trial. Gastroenterology 2019, 157, 440–450.e8.
- Svolos, V.; Hansen, R.; Nichols, B.; Quince, C.; Ijaz, U.Z.; Papadopoulou, R.T.; Edwards, C.A.; Watson, D.; Alghamdi, A.; Brejnrod, A.; et al. Treatment of Active Crohn’s Disease With an Ordinary Food-based Diet That Replicates Exclusive Enteral Nutrition. Gastroenterology 2019, 156, 1354–1367.e6.
- Suskind, D.L.; Lee, D.; Kim, Y.M.; Wahbeh, G.; Singh, N.; Braly, K.; Nuding, M.; Nicora, C.D.; Purvine, S.O.; Lipton, M.S.; et al. The Specific Carbohydrate Diet and Diet Modification as Induction Therapy for Pediatric Crohn’s Disease: A Randomized Diet Controlled Trial. Nutrients 2020, 12, 3749.
- Herrador-López, M.; Martín-Masot, R.; Navas-López, V.M. EEN Yesterday and Today … CDED Today and Tomorrow. Nutrients 2020, 12, 3793.
- Lewis, J.D.; Sandler, R.S.; Brotherton, C.; Brensinger, C.; Li, H.; Kappelman, M.D.; Daniel, S.G.; Bittinger, K.; Albenberg, L.; Valentine, J.F.; et al. A Randomized Trial Comparing the Specific Carbohydrate Diet to a Mediterranean Diet in Adults with Crohn’s Disease. Gastroenterology 2021, 161, 837–852.e9.
- Godny, L.; Reshef, L.; Pfeffer-Gik, T.; Goren, I.; Yanai, H.; Tulchinsky, H.; Gophna, U.; Dotan, I. Adherence to the Mediterranean diet is associated with decreased fecal calprotectin in patients with ulcerative colitis after pouch surgery. Eur. J. Nutr. 2020, 59, 3183–3190.
More
