Many obesity and diet-related diseases have been observed. Insulin resistance (IR), a state of tissue resistance to insulin due to its impaired function, is a common coexisting condition. The most important predisposing factors are excessive visceral fat and chronic low-grade inflammatory response. An additional disease that is often associated with IR is urolithiasis. The common feature of these two conditions is metabolic acidosis and mild inflammation. A patient diagnosed with IR and urolithiasis is a big challenge for a dietitian. It is necessary to check a thorough dietary history, make an appropriate anthropometric measurement, plan a full-fledged diet, and carry out the correct nutritional treatment. It is also essential to conduct proper laboratory diagnostics to plan nutritional treatment, which is often a big challenge for dietitians.
- insulin
- insulin resistance
- diet
- dietitian
- urolithiasis
1. Introduction
2. Insulin Resistance
IR is a condition of impaired insulin action by which glucose homeostasis is disturbed. As a result, tissue response to insulin is reduced while maintaining normal or elevated blood levels. Resistant tissues cannot properly metabolise glucose that remains in the blood. As a result, pancreatic β cells do not stop producing insulin. Excessive insulin synthesis leads to increased tissue resistance to insulin, forming a vicious circle. A simplified diagram of the vicious circle is shown in Figure 12. The chronic inflammatory response and low activity cause IR development [5][6][10][11][12][5,6,18,19,20]. The reasons for IR include (1) insufficient physical activity, (2) visceral fat level, (3) impact of some types of drugs, and (4) genetic factors. Moreover, it has been discovered that the development of IR can be associated with several conditions, such as cardiovascular diseases, non-alcoholic fatty liver disease, strokes, or polycystic ovary syndrome. IR significantly increases the risk of type 2 diabetes and metabolic syndrome [12][13][14][15][20,21,22,23].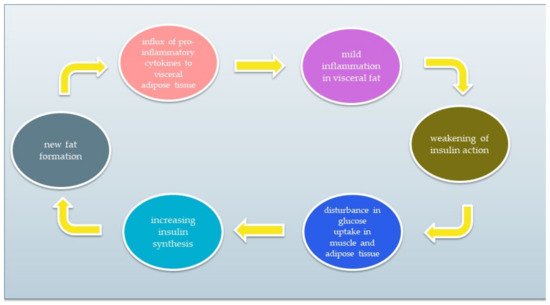
3. Urolithiasis
Urolithiasis is one of the oldest known diseases. Through archaeological research of Egyptian mummies, it has been proven that this disorder affected society in antiquity. It is characterised by insoluble deposits (stones) in the urinary tract formed due to the crystallisation of components present in the urine [17][51]. These stones often cause discomfort in the form of acute colic pain, nausea, and vomiting. They range in size from a few micrometres to a few centimetres. It is estimated that about 97% is in the kidneys and ureters, and 3% is in the bladder and urethra [18][52]. Thanks to technological advancement, rwesearchers now know that stones can consist of about 100 different chemical compounds and 80% of them mainly contain calcium oxalate (CaOx). Besides calcium oxalates, researcherswe usually find calcium phosphate and gout stones [19][40]. Treatment of urolithiasis usually involves surgical removal of the stones. Unfortunately, it does not remove the causes of their formation [18][52]. Scientific research indicates a relationship between the occurrence of urolithiasis with diet, lifestyle, and climate [18][19][20][40,41,52].4. Urolithiasis, Metabolic Acid, Inflammation, and Insulin Resistance
The mechanisms linking urolithiasis to the features of MS are not fully understood. Nevertheless, attention is often paid to the mild inflammation characteristic of MS, which also occurs in the presence of IR [21][53]. One of the most frequently studied inflammatory markers is interleukin-8 (IL-8/CXCL8). Its role in the development of neoplasms is often emphasised [22][54]. Its secretion is stimulated by hypoxia, reactive oxygen species, bacterial particles, and other cytokines such as IL-1, IL-6, or TNFα. The cells that secrete it are mainly leukocytes: blood monocytes, macrophages, epithelial and endothelial cells, and fibroblasts [22][23][54,55]. Suen et al. [24][56] focused on finding prognostic markers of urolithiasis and showed that the presence of urinary stones is associated with an inflammatory response, especially with increased levels of IL-8 in the urine. They suggest that IL-8 could be a marker as a screening test for urolithiasis. Wang et al. [25][57] showed that the values of pro-inflammatory cytokines, including IL-8 in plasma, decreased in patients after percutaneous nephrolithotripsy and suggested that IL-8 can be used as a predictive tool for patients after this procedure. Another proposed explanation is the association of low ammonium excretion with urine in MS patients, which causes acidification of the urine [20][26][41,48]. Systemic metabolic acidosis alters the concentration of various substances in the urine and may contribute to stone formation. The occurrence of diet-induced metabolic acidosis activates kidney compensatory mechanisms to correct the acid-base balance. Removal of non-metallised anions or excretion of ammonium ions can then occur. This decreases urine pH, causing changes in its composition, hypocitraturia, hypercalciuria, and nitrogen and phosphorus loss. This predisposes to the formation of urinary stones [27][58]. Research by Kim et al. [28][59] showed that higher glucose levels and HOMA-IR index characterised men with nephrolithiasis. Such changes were not observed in the female population. Factors that predispose to insulin resistance are conditions characteristic of metabolic acidosis, such as insulin resistance in skeletal muscles and increased secretion of glucocorticosteroids and cortisol. Studies suggest that even minor deviations in pH towards metabolic acidosis reduce the sensitivity of tissues to insulin [27][58]. It can therefore be assumed that among patients with IR, an increased tendency to develop urolithiasis can be expected and vice versa, due to similar mechanisms causing these diseases, such as the presence of systemic metabolic acidosis, MS features, and the presence of inflammatory mediators such as interleukin 6 (IL-6) or tumor necrosis alpha (TNFα).5. Diet—Insulin Resistance and Acidosis
Patients with IR usually do not achieve the desired dieting therapy results after using a traditional low-calorie diet. In this case, it is necessary to introduce modifications to improve carbohydrate metabolism and reduce tissue resistance to insulin. The selection of the right amount and type of carbohydrates is crucial to lowering excess insulin production, which affects lipid metabolism processes in cells [29][62]. In countries with highly processed food consumption, urolithiasis is more common in the population, both among adults and children [19][40]. Foods rich in animal protein increase the production of metabolic acid compounds in the body, such as hydrogen chloride or bisulfate, which may affect the body’s acid-base balance [30][31][32][33][34][66,67,68,69,70]. When these foods are consumed, the acids are buffered by the lung excretion of CO2 and the production of sodium salts. These salts are then excreted through the kidneys, mainly with ammonium. Bicarbonate resulting from buffering is returned to the plasma as a substitute for bicarbonate used to soften the acids. When the production of acidic compounds exceeds their excretion via the lungs and kidneys, the amount of bicarbonate in the plasma decreases and thus the blood pH decreases [34][70]. This mechanism is presented in Figure 2. Studies suggest that treatment of metabolic acidosis with bicarbonate increases insulin sensitivity; therefore, to correct the acid-base balance, it is recommended to significantly increase the amount of alkaline-forming vegetables and fruits in the diet [33][35][69,71]. It has been proven that people on a plant-based diet have substantially lower HOMA-IR values and blood glucose levels. It suggests that a diet rich in plant products and limiting animal foods may significantly affect the development of IR [36][72].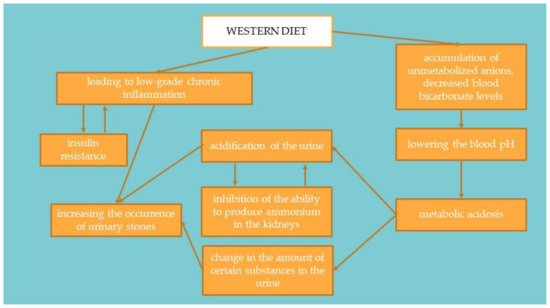
5.1. Carbohydrates
Scientific reports regarding the recommendations for the number of carbohydrates in the diet seem contradictory. They suggest that both a high-carbohydrate diet and a low-carbohydrate diet can benefit patients. Therefore, the number of carbohydrates in dietary therapy should be individually adjusted for each patient based on its effects. Too low an amount of carbohydrates can adversely affect the brain and initiate ketone bodies’ production; too much causes excessive insulin secretion and, consequently, no diet effects. Regardless of the chosen dietary direction, most carbohydrates consumed by the patient should be high-fibre vegetables and then grain products with a low glycemic index. The remaining amount should be filled with fruit and a low index. Berries are especially recommended, such as strawberries, raspberries, and blueberries. It should be emphasised to significantly reduce alcohol consumption, increasing blood glucose [37][38][39][40][65,73,74,75]. In addition, attention should be paid to the excessive use of sweeteners, which can significantly increase carbohydrate-induced insulin secretion [41][15].5.2. Proteins
If it seems justified to reduce the number of carbohydrates in the patient, then the amount of protein should be increased (about 15–20%). However, it should be remembered that too much animal protein causes acidification. Therefore, an appropriate amount of alkaline-forming vegetables and fruits should be selected to reduce the possibility of acidification of the organism [33][35][69,71]. The diet’s primary protein sources should be lean meat, unsweetened dairy products without additives (semi-fat products are recommended), legumes, and fatty marine fish containing large amounts of Omega-3 fatty acids. In lipid disorders, it is necessary to reduce red meat significantly. The addition of products including protein to meals with carbohydrates improves the body’s sensitivity to insulin; therefore, it is worth combining dishes, e.g., fruit and kefir cocktails or as additives sauces based on cream or yoghurt for carbohydrate dishes [42][43][76,77].5.3. Fats
Fats delay gastric emptying and absorption of nutrients, including glucose. Thanks to this, they prolong their metabolism. However, it should be pointed out that IR is usually accompanied by overweight or obesity, so the quality of fat taken by the patient is extremely important. Scientific reports indicate that the composition of fatty acids significantly influences IO’s development due to their role in the correct arrangement of cell membrane lipids. Maintaining an adequate omega-three and omega-six fatty acids ratio in the diet is recommended. Cold-pressed unrefined oils are best suited, for example, rapeseed oil or olive oil. ResearchWers should avoid products rich in saturated fatty acids, such as fatty meat, lard, and palm oil [44][45][11,78].6. Tips for Laying Diets
To properly balance the patient’s diet, follow the rules usually used during composing diets for people with diabetes. ItWe can be included, among others:-
eliminating juices and sweetened drinks, replacing them with water and tea (herbal, green, red)
-
choosing highly processed bread, e.g., rice cakes or crispbread, buying bread containing caramel, malt, or honey
- avoiding fruit in processed form, for example, juices, nectars, mousses, jams, because the sugar they contain is absorbed much faster than in the case of unprocessed fruits
-
properly composed meals—combine products with a higher amount of carbohydrates with products containing fat and protein—this will slow down the absorption of glucose into the blood
-
proper planning of meal times—eating regularly and at regular intervals.
- use of products containing large amounts of salt and preservatives, e.g., broth cubes, spice mixtures, flavour enhancers, Maggi
-
buying light products with low-fat content is often offset by an additional portion of sugar in the product.
