Leukodystrophies are genetic diseases primarily affecting the production, processing, or development of myelin in the central nervous system (CNS) and components of the white matter, such as oligodendrocytes and astrocytes. The broader term leukoencephalopathy refers to all white matter diseases, either hereditary or acquired, which can be due to neuronal or systemic pathologies.
2. Adult-Onset Leukodystrophy with Axonal Spheroids and Pigmented Glia (POLD, HDLS, ALSP): CRP Linked to Mutations in CSF1R, and LKENP (Leukoencephalopathy, Progressive with Ovarian Failure) Linked to Heterozygous Mutations in AARS2
2.1. Clinical Features
2.1.1. ALSP-CSFR1 or CRL
A large study comprising 90 families with the
CSFR1 mutation revealed the mean age at onset to be 43 years (range 18–78 years), mean age at death 53 years (range 23–84 years), with a mean disease of duration 6.8 years (range 1–29 years); clinical symptoms in women appear at a younger age
[7][36].
Behavioral and personality changes, memory impairment, executive dysfunction, and dementia are common symptoms. These are often accompanied by motor dysfunction including pyramidal signs (spasticity, hyperreflexia, hemiparesis, quadriparesis), sensory deficits, depression, and parkinsonian symptoms and signs such as gait impairment, rigidity, bradykinesia, postural instability, and tremor
[7][8][9][10][11][12][13][14][15][16][17][26,27,36,37,38,39,40,41,42,43,44]. Speech difficulty, non-fluent aphasia, and symptoms mimicking frontotemporal dementia behavioral variant (FTD-bv) have been described
[18][19][20][21][31,45,46,47]. Less common manifestations are corticobasal syndrome and stroke
[13][40]. Cerebellar and bulbar/pseudobulbar signs may occur at advanced stages, whereas seizures may appear at the onset of the disease. Peripheral neuropathy has been noted in some patients
[20][22][46,48]. Yet, whether peripheral neuropathy belongs to the CSF1R-related spectrum is an open question requiring further investigation
[20][46]. Involvement of the optic nerve is very rare
[23][49].
Considering the variability of clinical symptoms, misdiagnoses such as frontotemporal dementia, parkinsonism, multiple sclerosis, normal-pressure hydrocephalus, and vasculitis of the central nervous system, among others, are commonplace
[24][25][26][50,51,52]. Differential diagnosis covers several entities, mainly adult-onset leukodystrophies
[2][18][2,31].
2.1.2. ALSP-AARS2 or LKENP
Dallabona et al.
[27][33] described another group of patients with progressive leukoencephalopathy starting in childhood or in early adulthood, characterized by progressive gait ataxia, tremor, spasticity, dystonia, dysarthria, and cognitive decline. The affected women presented with ovarian failure and amenorrhea, followed by neurodegeneration, or presented ovarian failure during the progression of the neurological disorder. The investigation of mitochondrial function in two patients identified cytochrome c oxidase deficiency. Ragged-red fibers were absent. None of the patients had signs of cardiomyopathy. Brain MRI showed leukoencephalopathy with the involvement of left-right connections, descending tracts, and cerebellar atrophy. Next-generation sequencing revealed compound heterozygous mutations in
AARS2, which encodes mitochondrial alanyl-tRNA synthetase, in both patients. Functional studies in yeast confirmed the pathogenicity of the mutations in one patient
[27][33]. Additional cases of leukoencephalopathy linked to
AARS2 mutations have been reported
[28][53].
2.2. Radiological Findings
Atrophy and increased signal in the white matter, thinning of the corpus callosum, abnormal signaling of the pyramidal tracts, and enlarged ventricles are seen on MRI. The subcortical and periventricular white matter shows T2-weighted and FLAIR hyper-intensities with no gadolinium-enhanced lesions; these hyperintensities are usually diffuse but they can be confluent, with asymmetric distribution. Calcifications in the white matter are found in about one half of cases; calcifications in the frontal periventricular white matter are common in cases linked to
CSFR1 mutations (CRL), but they are absent in cases linked to
AARS2 mutations (LKENP)
[7][8][11][12][14][17][27][29][30][31][32][33][34][35][36][24,26,33,36,38,39,41,44,54,55,56,57,58,59,60].
In CT images, calcifications mainly involve the frontal white matter adjacent to the anterior horns of the lateral ventricles and the parietal subcortical white matter; calcifications have a symmetrical, “stepping stone” appearance in the pericallosal regions
[37][61]. Thin-slice CT techniques are necessary to detect small calcifications
[14][41]. The cerebellum and the brain stem appear normal.
MR spectroscopy shows changes in metabolite concentrations not only in patients with HDLS linked to
CSFR1 mutation and also in asymptomatic
CSF1R mutation carriers
[38][62].
2.3. Neuropathology
The characteristic lesions are: (a) bilateral and confluent demyelination of the cerebral white matter sparing the cortico-subcortical U fibers, and involvement of the corpus callosum and internal capsule; (b) axonal damage with spheroids in the white matter; (c) infiltration of macrophages filled with neutral lipids; and (d) cytoplasmic deposits in macrophages, astrocytes, and oligodendrocytes stained with periodic acid–Schiff, Sudan black, and Klüver–Barrera that are autofluorescent in paraffin sections
[11][12][13][14][19][39][40][11,12,38,39,40,41,45]. Axonal damage is best seen with silver-based methods and immunohistochemistry with antibodies against neurofilaments, β-amyloid precursor protein, and ubiquitin. Electron microscopy reveals that spheroids are mainly composed of neurofilaments; less frequently, they also contain microtubules, mitochondria, and altered mitochondria, together with granular material
[41][42][20,63]. Pigments contain electron-dense granular material with lamellar or fingerprint arrangement reminiscent of ceroid-lipofuscin
[39][40][41][43][11,12,20,25] (
Figure 1 and
Figure 2). Characteristic lesions are also seen in cerebral biopsy samples.
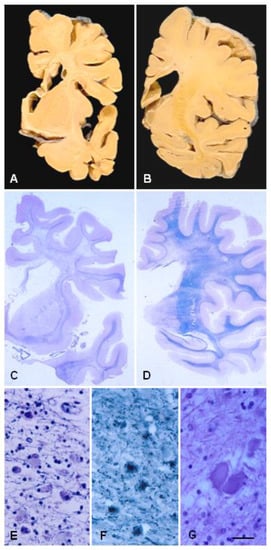
Figure 1. ALSP. (A,B) Coronal sections of the brain show atrophy of the cerebral white matter more markedly in the anterior poles than in the posterior regions, atrophy of the corpus callosum, and enlargement of the lateral ventricles. (C,D) Hemispheric sections stained with Klüver–Barrera show severe myelin loss of the centrum semi-oval, corpus callosum, and internal capsule, and preservation of the short cortico-subcortical U-fibres. (E,F) Astrocytic gliosis and macrophages filled with pigment in the affected white matter. (G) Axonal ballooning. Paraffin sections, (E–G), bar = 25 μm.
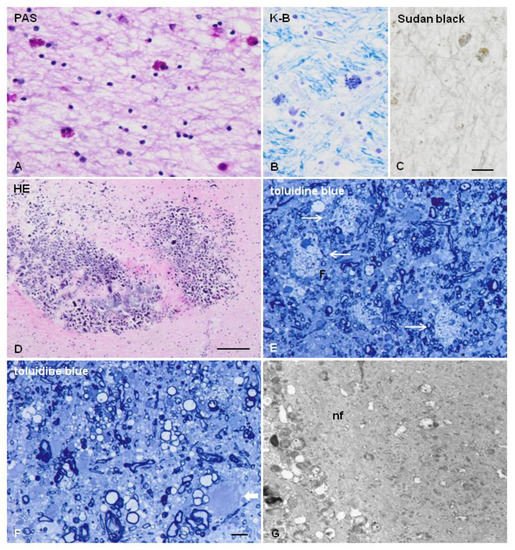
Figure 2. ALSP. (A–C) Glial cells filled with pigment as seen with periodic acid–Schiff (PAS) (A), Klüver–Barrera (K-B) (B), and Sudan black (C) staining in the frontal white matter. (D) Microcalcifications in the frontal white matter near the lateral ventricle. (E,F) Semi-thin sections stained with toluidine blue showing loss of myelin, macrophages filled with neutral lipids (thin arrows in (E)), and axonal ballooning (thick arrow in (F)). (G) Axonal ballooning filled with neurofilaments (nf) in the white matter. (A–D), formalin-fixed samples, paraffin sections; (E–G), samples fixed with glutaraldehyde, post-fixed with osmium tetraoxide, and embedded in Araldite; ultra-thin sections stained with uranyl acetate and lead citrate. (A–C), bar = 25 μm; (D) = 100 μm; (E–F) = 10 μm.
The abundance of spheroids and pigmented glia varies from one case to another and with disease progression
[10][43][44][45][46][25,37,64,65,66]. White matter lesions have been categorized in three stages: (1) white matter with numerous spheroids in a background of well-myelinated fibers, (2) moderate loss of myelinated fibers with a sparse-to-moderate number of spheroids, and (3) leukodystrophy pattern of confluent axonal and myelin loss
[47][67]. This pattern is in line with pioneering observations noting that: (i) the formation of spheroids is an early event in ALSP, (ii) spheroids are more abundant in areas of partial demyelination than in areas of extensive demyelination, and (iii) the loss of oligodendrocytes occurs in regions of extensive demyelination but not partial demyelination
[19][45]. Similar lesion-based stages have been proposed, with two additional conclusions: (i) shape, density, and subsets of microglia change with stage progression, and (ii) an increase in IBA-1, CD-68, CD-163-, and CD-204-immunoreactive cells precedes the loss of axons
[46][66]. Neuropathological features were reported in a non-affected
CSF1R carrier who died of tuberculosis. Patchy demyelination and axonal loss predominate in the subcortical white matter, whereas pigmented microglial cells are distributed throughout the white matter preceding myelin and axonal loss
[48][18]. Interestingly, activated microglia are spatially restricted, rather than diffusely distributed, in ADLS cases bearing
CSF1R mutations
[14][41]. Quantitative studies show a predominance of axonal spheroids and axonal depletion in areas with an almost complete absence of ramified microglia, thus suggesting that loss of microglial ramification, indicative of activation, precedes axonal spheroid formation
[47][67].
The caudate, putamen, thalamus, hypothalamus, hippocampus, substantia nigra, nuclei of the brain stem, and cerebellar cortex are unaffected or only very mildly affected
[18][31].
Axonal swellings have been noted in skin nerves
[41][20].
3. Polycystic Membranous Lipomembranous Osteodysplasia with Sclerosing Leukoencephalopathy (PLOSL; Nasu–Hakola Disease)
3.1. Clinical Features
The disease usually manifests in the third decade by multiple cyst-like lesions and loss of bone trabeculae, causing pain and tenderness, mainly in feet and ankles; fractures following minor accidents are common. The osseous stage is followed by a progressive neurological disease consisting of abnormal personality and behavior, episodes of depression and euphoria, loss of social inhibition, memory deficits, and apathy, together with spasticity, postural dyspraxia, and involuntary movements. Urinary incontinence, loss of libido, and sexual impotence are early symptoms in some cases. Seizures may be present. Patients die in a vegetative state before the age of 50 years
[49][50][51][52][53][54][55][56][57][58][59][60][61][111,112,115,116,117,118,119,120,121,122,123,124,125]. Due to the first appearance of osseous lesions in most cases, neuropsychological tests and functional neuroimaging can be useful in identifying subclinical bone alterations in the early stages of NHD
[62][63][126,127]. Progressive spastic paraplegia and dementia without skeletal symptoms, but accompanied by polycystic osteodysplasia following X-ray examination, are clinical manifestations in some subjects
[62][126]. In some cases, skeletal symptoms are not apparent, and patients complain of behavioral and neurological deficits. NHD cases without apparent skeletal symptoms occur in patients with mutations in
TREM2, but not
TYROBP [64][65][66][67][68][128,129,130,131,132]. Osseous pathology is not always present or diagnosed
[57][61][69][121,125,133]. Radiological examination of bones is recommended in patients with neurological deficits clinically suggestive of NHD.
3.2. Radiological Examination
In NHD, cyst-like lesions are typically found in the carpal and tarsal bones and in the fingers
[51][57][70][115,121,134].
Brain CT scans show cerebral atrophy with enlargement of the frontal regions of the lateral ventricles and calcifications in the globus pallidus. MRI reveals diffuse high density in the cerebral white matter and reduced density in the basal nuclei on T2-weighed images; white matter lesions extend from the periventricular areas to the periphery, sparing the cortico-cortical arcuate fibers
[56][57][71][72][73][74][75][76][113,120,121,135,136,137,138,139]. Hypoperfusion of the grey matter has been noted in SPECT and PET studies
[77][78][140,141].
3.3. General Pathology
Bone lesions are membrane-cystic lesions that destroy the osseous tissue. Membranes are convoluted, eosinophilic, PAS-positive, and autofluorescent; they contain carbohydrates, phospholipids, fatty acid crystals, hydroxyapatite crystals, and collagen. Under electron microscopy, the membranes mainly form tubular and saccular structures and contain granular material. Cysts are filled with triglycerides. In addition, the neighboring blood vessels show reduced lumen, enlargement of the inner elastic membrane, and degeneration of the muscular layer
[56][79][80][81][82][83][114,120,142,143,144,145]. Examination of a transiliac bone biopsy sample from one patient revealed disordered lamellar collagen fibril arrangement and increased matrix mineralization, pointing toward the involvement of osteoclast defects in this condition
[84][146].
In addition to the bones, membranous lipodystrophy appears as a form of degeneration of the adipose tissue
[82][144]. Several tissues are also affected, such as the cutaneous and perivisceral adipose tissue, bone marrow, alveolar septa of the lungs, and rectal mucosa
[56][62][85][120,126,147].
3.4. Neuropathology
The central nervous system in NHD is decreased in size and has a reduced consistency of the cerebral white matter, atrophy of the frontal and temporal lobes, and dilated ventricles. The globus pallidus presents with brownish discoloration on macroscopic examination. Histological sections processed for myelin staining exhibit generalized symmetrical demyelination of the cerebral white matter with preservation of the arcuate subcortical fibers, corpus callosum, and white matter of the cerebellum. There is marked astrogliosis, but with the discrete presence of macrophages filled with sudanophilic material, and the absence of inflammatory infiltration. Axonal degeneration accompanied by axonal spheroids is common. Axonal spheroids contain neurofilaments, mitochondria, vesicles, and granular material
[61][62][86][87][125,126,148,149]. The blood vessels in the white matter have increased thickness and multi-layering of the basement membrane
[56][88][120,150].
The grey matter has less notable pathology
[87][149]. The globus pallidus shows variable neuron loss and astrocytic gliosis, and numerous calcospherites containing calcium, iron, and glycoprotein matrix
[62][76][86][87][89][126,139,148,149,151]. The thalamus and hippocampus show a discrete neuronal loss in most cases. Marked neuronal loss and astrocytic gliosis in the caudate nucleus, putamen, substantia nigra, and thalamus (particularly in the dorsomedial and anterior nucleus) are found in some patients
[87][149]. Severe thalamic degeneration occurs only rarely
[90][152].
Alzheimer’s disease pathology (neurofibrillary tangles and senile plaques) is as common in NHD as in the control population
[91][153]. However, a recent report described a 51-year-old female with NHD, bearing a homozygous mutation (Q33X) of the
TREM2 gene, and showing spots of neurofibrillary tangle pathology in the neocortex, but sparing the mesial temporal structures on post-mortem examination
[92][154].
The percentage of short carbon chain non-hydroxyl fatty acids of sulfatide and the percentage of palmitic acid of ganglioside in the cortex in NHD were both increased in comparison to controls
[93][155].
Peripheral neuropathy with axonal and segmental degeneration has been reported in a few cases
[62][94][126,156].