Among the many oncology therapies, few have generated as much excitement as CAR-T. The success of CAR therapy would not have been possible without the many discoveries that preceded it, most notably, the Nobel Prize-winning breakthroughs in cellular immunity. However, despite the fact that CAR-T already offers not only hope for development, but measurable results in the treatment of hematological malignancies, CAR-T still cannot be safely applied to solid tumors. The reason for this is, among other things, the lack of tumor-specific antigens which, in therapy, threatens to cause a lethal attack of lymphocytes on healthy cells. In the case of hematological malignancies, dangerous complications such as cytokine release syndrome may occur. Scientists have responded to these clinical challenges with molecular switches.
1. Nobel Prizes in Cell-Mediated Immunity Research and CAR
Immunotherapy of tumors is one of the most rapidly developing fields of medicine. Although it is associated with modern treatment, its origins can be found at the end of the 19th century. At that time, a case was described in which osteosarcoma regressed after contracting erysipelas. Shortly thereafter, William Bradley Coley made an effective attempt to treat tumors with the use of bacteria causing erysipelas
[1]. Although this was largely chance-based, it exploited complex immunological mechanisms. An example of the most advanced immuno-oncology therapy is the concept of chimeric antigen receptor (CAR). The classical CAR consists of an epitope-recognizing region, namely, a single-chain variable fragment (scFv), along with a costimulatory domain, most commonly CD28 or 4-1BB, and a CD3ζ signaling domain
[2]. These genetically modified lymphocytes are used to destroy treatment-resistant blood tumors. However, for CARs to be designed, ample research over many years, was necessary. Among such research, the Nobel Prize-winning discoveries in the field of cellular immunity might be consider as some of the most important. Ralph M. Steinman (Nobel Prize 2011) discovered dendritic cells that, through the B7 protein, activate the CD28 domain present on T lymphocytes
[3]. In CAR-T, CD28 became the classic chimeric receptor element
[2]. Ralph M. Steinman shared the award with Bruce A. Beutler and Jules A. Hoffmann, whose work on Toll-like receptors, characteristic of macrophages, contributed to the use of the costimulatory molecule MyD88 (part of the TLR signaling pathway) as a strategy to increase CAR potency
[4][5][4,5]. The discovery of macrophages and phagocytosis is attributed to Ilya Ilyich Mechnikov who, together with Paul Ehrlich received a Nobel Prize in 1908, made possible the use of CAR macrophages (CAR-M) as an alternative to CAR-T
[6][7][6,7]. Another key factor was the observation of MHC-restricted antigen recognition, a phenomenon that makes T cell receptors (TCRs) require not only an antigen but also an MHC molecule to activate them (Doherty and Zinkernagel, 1996)
[8]. The chimeric antigen receptor is designed to recognize antigens independently of the MHC, which greatly increases its effectiveness in killing, especially when the tumor cell lacks an MHC
[9]. The MHC is also important in distinguishing between self and foreign cells. Sir Frank Macfarlane Burnet, along with Peter Brian Medawar, received awards in 1960 for understanding how cellular immunity, although effective against foreign antigens, does not destroy host tissues
[10][11][10,11]. In addition, the practical application of knowledge of immunology in the first transplants performed by Joseph E. Murray and E. Donnall Thomas was important (awarded Nobel Prizes in 1990)
[12][13][12,13]. In relation to CAR-T, these discoveries are particularly relevant because this therapy is an example of cell transplantation and can only be administered autologously. However, work is also underway on cells for allogeneic administration
[14]. Another important line of Nobel Prize-winning research was on immune checkpoint blockade (James P. Allison and Tasuku Honjo in 2018). Receptors for PD-L1 are found, among others, on T cells, and serve to deactivate these cells. Tumors take advantage of this fact by increasing the expression of ligands for PD-1, thus disabling lymphocytes. The use of checkpoint inhibitors such as PD-L1 and CTLA-4 has shown suitable results in the treatment of many cancers.
[15][16][15,16]. CAR-T can be subjected to modifications involving the knock-out of genes for checkpoint receptors, thus making them insensitive to tumor suppression without the separate addition of anti-PD-1 antibodies
[17].
With these advantages, CARs have achieved undisputed success and have already been registered as a treatment for five blood malignancies. However, CAR therapy is being studied for more than just hematological malignancies and more than just T cells. Natural killer (NK) cells also seem to be attractive since they are not sensitive to suppression signals from Tregs. Additionally, natural killers are able to secrete tumor necrosis factor and interferon-gamma. NK cells are also attractive in the context of off-the-shelf therapies because they do not naturally cause graft-versus-host disease
[18]. CARs have also been used for solid tumors such as glioblastoma, breast cancer, and prostate cancer
[19][20][21][19,20,21]. Moreover, some studies indicate the possibility of using CAR-T to treat autoimmune diseases such as rheumatoid arthritis or lupus
[22][23][22,23] and even to combat viral infections, including HIV infection and SARS-CoV-2
[24][25][24,25].
2. Dark Side of CAR Therapy
Despite the prevailing optimism, CARs also have a dark side, represented by potentially serious side effects. Among the more serious adverse effects that can lead to death are cytokine release syndrome (CRS), which may affect up to 92% of patients, and neurotoxicity (ICANS)
[26][27][28][26,27,28]. In the case of solid tumors, the problem is also the lack of completely specific antigens, which makes possible a fatal on-target off-tumor attack with CAR-T application. In blood tumors, despite the relative specificity of the CD19 receptor, bone marrow aplasia can occur resulting in death or requiring stem cells transplantation
[29][30][29,30]. Despite the developed treatment in the form of tocilizumab (an anti-IL-6 receptor antibody), there are cases where the patient dies regardless of its application
[31][32][31,32]. Besides the mentioned tocilizumab, there is also treatment with siltuximab (anti-IL-6 antibody)
[33]. As well as blocking the action of IL-6, IL-1 can also be blocked. Treatment with anakinra, which binds to IL-1, has shown good results in both ICANS and CRS contexts
[34]. It is also possible to block IL-1 secretion at the macrophage level by blocking inflammasome formation
[35]. Despite some achievements of this type of therapy, it is a symptomatic rather than causal treatment. To increase the possibility of limiting the side effects of therapy, especially on-target off-tumor attacks, a mechanism is needed to control the action of CARs. One potential method to enable elimination of the CAR-T in vivo could be the use of anti-CARs, namely other CAR-Ts, designed to kill previously administered lymphocytes
[36]. However, this is an expensive solution that requires prior preparation of appropriate anti-CARs, as the long production of CARs prevents their rapid delivery within a reasonable time for the patient.
A solution to the problem of a lack of control may be the switches, which together with the CAR-containing construct, are implemented in lymphocytes. Thanks to their design, they make it possible to control the activity of CAR by administering appropriate molecules or even light or temperature. They enable the control of CAR-T activity in vivo, i.e., in the patient’s body, which means that lymphocyte deactivation can be ordered at the onset of symptoms or, in the case of the development of effective markers (e.g., MCP-1), even before the occurrence of complications
[37].
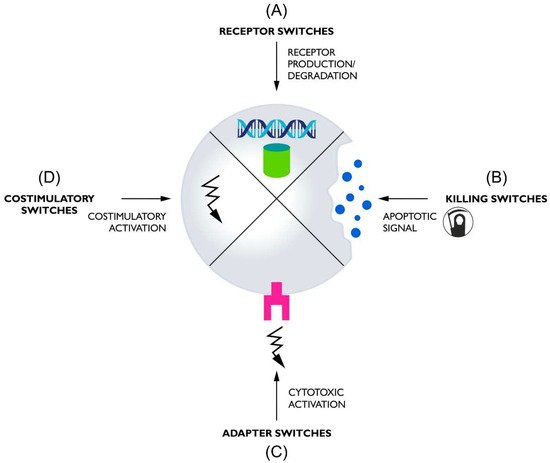
Switches used in CAR-T can, based on their functions, be divided into four basic groups: (1) receptor switches that control the formation or degradation of CAR protein, (2) killing switches that regulate CAR-T activity by inducing lymphocyte apoptosis, (3) adapter switches that require a molecule linking CAR to antigens to function, and (4) costimulatory switches that provide an additional regulated amplifying signal (Figure 1).
Figure 1. The diagram shows the general mechanism of the four classes of switches used to control CARs. (A) Receptor switches oversee the presence of the CAR protein in the cell by regulating its formation or elimination. (B) Killing switches control CAR-T activity by killing cells through apoptosis, triggered by a control molecule. (C) Adapter switches control receptor activation through a molecule mediating contact between CAR and the tumor antigen. (D) Costimulatory switches provide additional costimulation if appropriately activated.