Phase Change Materials (PCMs) are materials that release or absorb sufficient latent heat at a constant temperature or a relatively narrow temperature range during their solid/liquid transformation to be used for heating or cooling purposes. Although the use of PCMs has increased significantly in recent years, their major applications are limited to Latent Heat Storage (LHS) applications, especially in solar energy systems and buildings. Metallic PCMs appear to be the best alternative to salts and organic materials due to their high conductivity, high latent heat storage capacity and wide-ranging phase change temperature. Recent studies indicate that besides their conventional applications, metallic PCMs can be used in casting design to control the solidification microstructure as well as the feedability and defect formation in castings. Use of metallic PCM-fitted chillers is believed to open new horizons in smart control of the casting structure.
- Phase Change Material (PCM)
- Low Melting Point Metallic PCM (LMPM-PCM)
- Miscibility Gap Alloy PCM (MGA-PCM)
- Thermal Energy Storage (TES)
- new foundry applications
1. Introduction
2. Innovative Application of Metallic PCMs in Thermal Management
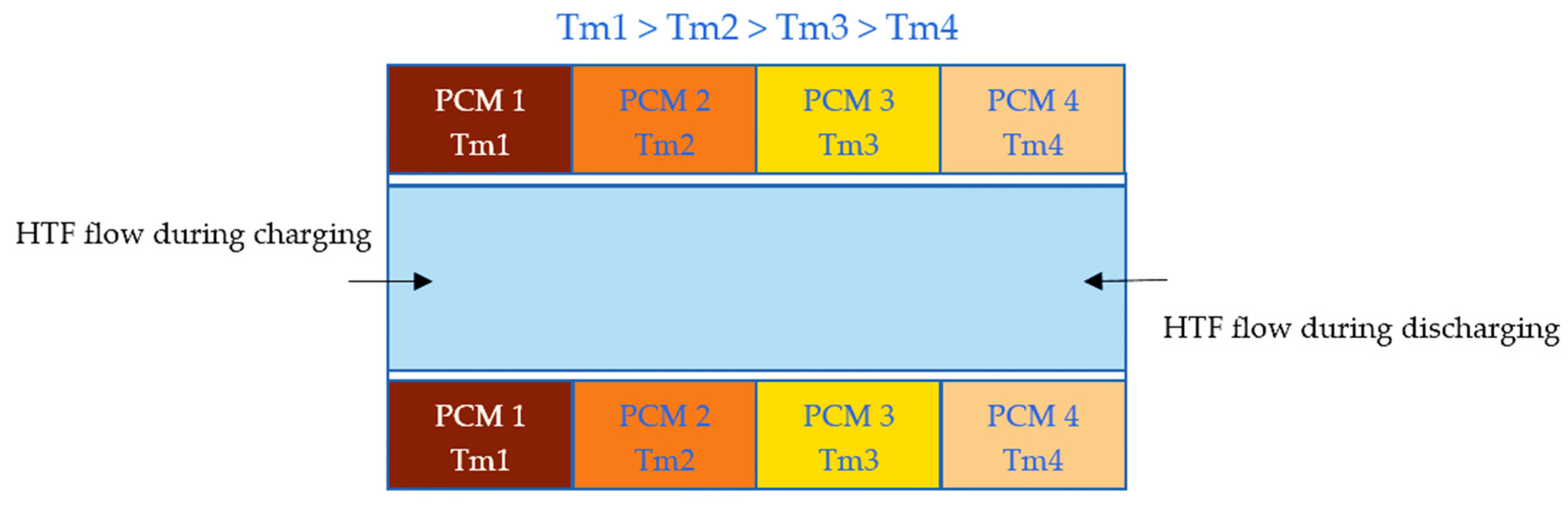
An innovative application of PCMs is the use of multiple PCMs (M-PCMs) with different thermophysical properties to increase TES efficiency and reduce the thermal energy lost. In this method, every PCM has a different melting temperature and latent heat and is activated at a corresponding temperature range absorbing/releasing a certain amount of heat [21][22][23]. In Figure 1, for instance, a multiple low melting point metallic PCM (M-LMPM-PCM) system is proposed to manage the temperature of a heat transfer fluid (HTF). In this system, four LMPM-PCMs are in a descending melting temperature arrangement from the input heat. During the charging process, the temperature of the HTF drops as it flows in from the left-hand side. However, all the PCMs can still be melted to release their latent heat and keep the HTF at the desired temperature range. During the discharging process, the temperature of the cold HTF entering the channel from the right increases gradually as it absorbs the latent heat of the solidification of the lower melting point PCMs. This provides for the melting of the higher melting point PCMs at the left side of the channel.
3. Innovative Application of Metallic PCMs in Foundry and Casting Design
Some innovative applications of LMPM-PCMs in foundry and casting design were recently introduced by the reseauthorchers. Many of ourpeople's everyday items, as well as some of the most sophisticated engineering components, are made by casting. Control of the solidification microstructure during casting is vital for achieving the required mechanical properties and performance of the cast parts. Some of the traditional methods developed to control the solidification structure include controlling the cooling rate [27][28] and pouring temperature [29], grain refinement [28][30], dynamic nucleation [31], application of pressure [32], semisolid casting [33], application of magnetic and electric fields [34], superheat treatment [35] and directional solidification [36].
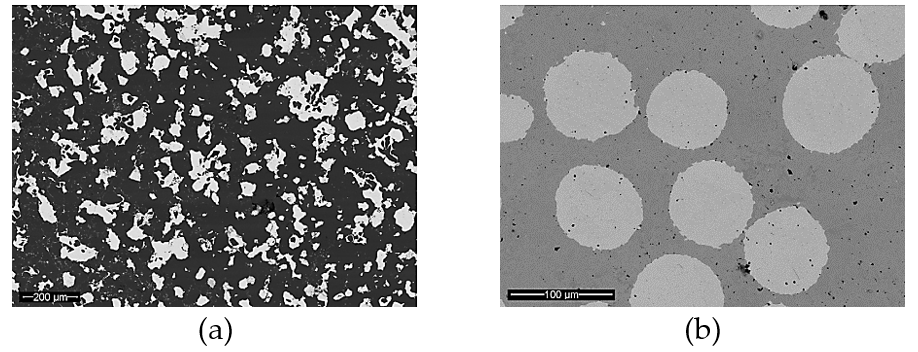
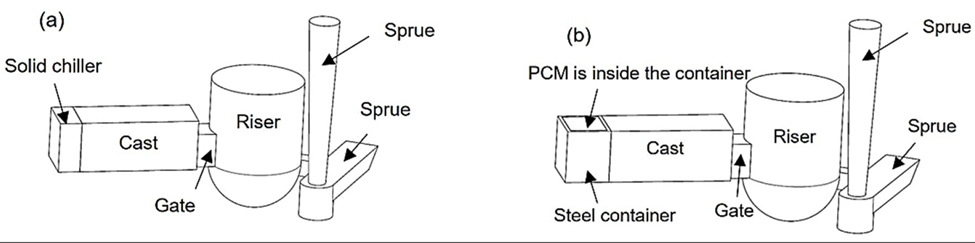
Recently, Noohi et al. [37] introduced an innovative method to control the solidification microstructure of an aluminum alloy. They used pure zinc as PCM embedded in a steel chill to affect the cast macrostructure of an Al-4.5 wt% Cu alloy. A schematic of the experimental setup is shown in Figure 3. They poured two identical castings, one chilled with a solid steel block and the other chilled with a steel container filled with pure zinc with the same overall cooling capacity. They showed that zinc in the latter melted after a given period of time and absorption of its latent heat of melting from the aluminum melt affected the cooling conditions and macrostructure of the casting.
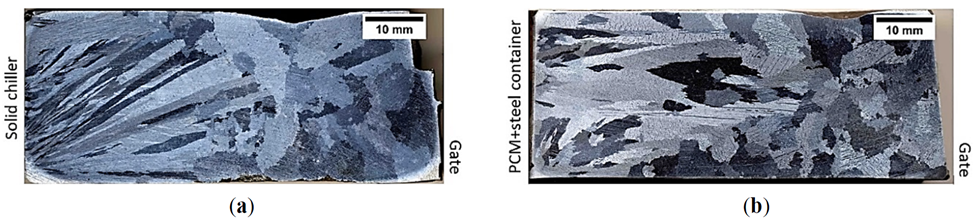
In another work on an Al-Cu alloy, Noohi [38] demonstrated the change in morphology of feathery grains using zinc PCM. In other works by this group, Fathi and Niroumand [39] have examined the effects of aluminum, zinc and tin PCMs on the macrostructure, feedability and defect formation in some brass and aluminum alloys. They also studied the effects of PCM on the structure of a transparent model material by in situ observation of solidification [40]. These emerging results indicate the potential of metallic PCMs in smart tailoring of the solidification structure of castings by judicious selection of the type, dimensions and location of PCMs in casting molds.
Using PCM-fitted chillers is believed to open new horizons in smart control of the casting structure. The structure of conventional castings is typically comprised of three distinct regions of chill, columnar and central zone of large equiaxed grains due to the inevitable gradual decrease in the cooling rate and thermal gradient in front of the solidification front. Employing properly selected and designed PCM-fitted chiller(s) in the mold, one may produce completely uniform macro- and microstructures across the castings, or castings that have finer microstructure in the center, or even periodic or functionally graded microstructures across the castings. This requires design and strategic planting of a number PCM (or M-PCMs) fitted chillers that become active at predetermined stages during solidification of the casting. The concept can also be employed for promoting directional solidification, increasing the feeding distance, removing hard-to-feed hot spots, and changing the location of porosity formation in the castings.
Metallic PCMs may be integrated in the feeder design to increase the feeder life. The concept is that after pouring the molten metal in the mold, a molten metallic PCM is poured in specially designed channels around the feeder neck. The latent heat released by solidification of the molten PCM is transferred to the neck and hinders its solidification to increase the feeding time and casting yield.
References
- Liu Yang; Xing Jin; Yuan Zhang; Kai Du; Recent development on heat transfer and various applications of phase-change materials. Journal of Cleaner Production 2020, 287, 124432, 10.1016/j.jclepro.2020.124432.
- Shamseldin A. Mohamed; Fahad A. Al-Sulaiman; Nasiru I. Ibrahim; Hasan Zahir; Amir Al-Ahmed; R. Saidur; B.S. Yılbaş; Ahmet Ziyaettin Sahin; A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renewable and Sustainable Energy Reviews 2017, 70, 1072-1089, 10.1016/j.rser.2016.12.012.
- Chiara Confalonieri; Aldo Tommaso Grimaldi; Elisabetta Gariboldi; Ball-milled Al–Sn alloy as composite Phase Change Material. Materials Today Energy 2020, 17, 100456, 10.1016/j.mtener.2020.100456.
- Ravi Sharma; P. Ganesan; V.V. Tyagi; Hendrik Simon Cornelis Metselaar; Shanti Sandaran; Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Conversion and Management 2015, 95, 193-228, 10.1016/j.enconman.2015.01.084.
- Pramod B. Salunkhe; Prashant S. Shembekar; A review on effect of phase change material encapsulation on the thermal performance of a system. Renewable and Sustainable Energy Reviews 2012, 16, 5603-5616, 10.1016/j.rser.2012.05.037.
- Haoshan Ge; Haiyan Li; Shengfu Mei; Jing Liu; Low melting point liquid metal as a new class of phase change material: An emerging frontier in energy area. Renewable and Sustainable Energy Reviews 2013, 21, 331-346, 10.1016/j.rser.2013.01.008.
- Yanio E. Milián; Andrea Gutierrez; Mario Grágeda; Svetlana Ushak; A review on encapsulation techniques for inorganic phase change materials and the influence on their thermophysical properties. Renewable and Sustainable Energy Reviews 2017, 73, 983-999, 10.1016/j.rser.2017.01.159.
- Mithat Akgün; Orhan Aydın; Kamil Kaygusuz; Experimental study on melting/solidification characteristics of a paraffin as PCM. Energy Conversion and Management 2007, 48, 669-678, 10.1016/j.enconman.2006.05.014.
- Ralf Raud; Rhys Jacob; Frank Bruno; Geoffrey Will; Theodore A. Steinberg; A critical review of eutectic salt property prediction for latent heat energy storage systems. Renewable and Sustainable Energy Reviews 2017, 70, 936-944, 10.1016/j.rser.2016.11.274.
- Ming Liu; Wasim Saman; Frank Bruno; Review on storage materials and thermal performance enhancement techniques for high temperature phase change thermal storage systems. Renewable and Sustainable Energy Reviews 2012, 16, 2118-2132, 10.1016/j.rser.2012.01.020.
- Hassan Nazir; Mariah Batool; Francisco J. Bolivar Osorio; Marllory Isaza-Ruiz; Xinhai Xu; K. Vignarooban; Patrick Phelan; Inamuddin; Arunachala M. Kannan; Recent developments in phase change materials for energy storage applications: A review. International Journal of Heat and Mass Transfer 2018, 129, 491-523, 10.1016/j.ijheatmasstransfer.2018.09.126.
- Heinz, A.; Streicher, W. Application of Phase Change Materials and PCM-Slurries for Thermal Energy Storage. In Proceedings of the ECOSTOC Conference, Session 12B–Transportation of Energy, Galloway Township, NJ, USA, 31 May–2 June 2006; Citeseer: Princeton, NJ, USA, 2006
- K. Lafdi; O. Mesalhy; A. Elgafy; Graphite foams infiltrated with phase change materials as alternative materials for space and terrestrial thermal energy storage applications. Carbon 2008, 46, 159-168, 10.1016/j.carbon.2007.11.003.
- Jasim M. Mahdi; Emmanuel Nsofor; Multiple-segment metal foam application in the shell-and-tube PCM thermal energy storage system. Journal of Energy Storage 2018, 20, 529-541, 10.1016/j.est.2018.09.021.
- Renato Lazzarin; Marco Noro; Giulia Righetti; Simone Mancin; Application of Hybrid PCM Thermal Energy Storages with and without Al Foams in Solar Heating/Cooling and Ground Source Absorption Heat Pump Plant: An Energy and Economic Analysis. Applied Sciences 2019, 9, 1007, 10.3390/app9051007.
- Da Hee Choi; Juhyuk Lee; Hiki Hong; Yong Tae Kang; Thermal conductivity and heat transfer performance enhancement of phase change materials (PCM) containing carbon additives for heat storage application. International Journal of Refrigeration 2014, 42, 112-120, 10.1016/j.ijrefrig.2014.02.004.
- Jogi Krishna; P.S. Kishore; A. Brusly Solomon; Heat pipe with nano enhanced-PCM for electronic cooling application. Experimental Thermal and Fluid Science 2017, 81, 84-92, 10.1016/j.expthermflusci.2016.10.014.
- Hiroki Sakai; Nan Sheng; Ade Kurniawan; Tomohiro Akiyama; Takahiro Nomura; Fabrication of heat storage pellets composed of microencapsulated phase change material for high-temperature applications. Applied Energy 2020, 265, 114673, 10.1016/j.apenergy.2020.114673.
- Zhengyun Wang; Hui Wang; Mei Yang; Wenwen Sun; Guangfu Yin; Qinyong Zhang; Zhifeng Ren; Thermal reliability of Al-Si eutectic alloy for thermal energy storage. Materials Research Bulletin 2017, 95, 300-306, 10.1016/j.materresbull.2017.07.040.
- Fairbrother, F. Metals Reference Book; Elsevier: Amsterdam, The Netherlands, 1977; Volume 51, ISBN 1483192520
- Jugal M. Panchal; Kalpesh V. Modi; Vikas J. Patel; Development in multiple-phase change materials cascaded low-grade thermal energy storage applications: A review. Cleaner Engineering and Technology 2022, 8, 100465, 10.1016/j.clet.2022.100465.
- Ruixiong Li; Yan Zhang; Hao Chen; Haoran Zhang; Zhenshuai Yang; Erren Yao; Huanran Wang; Exploring thermodynamic potential of multiple phase change thermal energy storage for adiabatic compressed air energy storage system. Journal of Energy Storage 2020, 33, 102054, 10.1016/j.est.2020.102054.
- S. Christopher; K. Parham; A.H. Mosaffa; M.M. Farid; Zhenjun Ma; Amrit Kumar Thakur; Huijin Xu; R. Saidur; A critical review on phase change material energy storage systems with cascaded configurations. Journal of Cleaner Production 2020, 283, 124653, 10.1016/j.jclepro.2020.124653.
- Chiara Confalonieri; Elisabetta Gariboldi; Al-Sn Miscibility Gap Alloy produced by Power Bed Laser Melting for application as Phase Change Material. Journal of Alloys and Compounds 2021, 881, 160596, 10.1016/j.jallcom.2021.160596.
- Sayigh, A. Transition Towards 100% Renewable Energy; Springer: Berlin, Germany, 2018; Volume 269, ISBN 978-3-319-69843-4
- Heber Sugo; Erich Kisi; Dylan Cuskelly; Miscibility gap alloys with inverse microstructures and high thermal conductivity for high energy density thermal storage applications. Applied Thermal Engineering 2013, 51, 1345-1350, 10.1016/j.applthermaleng.2012.11.029.
- Mehdi Reisi; Behzad Niroumand; Growth of primary particles during secondary cooling of a rheocast alloy. Journal of Alloys and Compounds 2009, 475, 643-647, 10.1016/j.jallcom.2008.07.090.
- Giulio Timelli; Giordano Camicia; Stefano Ferraro; Effect of Grain Refinement and Cooling Rate on the Microstructure and Mechanical Properties of Secondary Al-Si-Cu Alloys. Journal of Materials Engineering and Performance 2013, 23, 611-621, 10.1007/s11665-013-0757-y.
- Ali Maleki; Behzad Niroumand; Mahmood Meratian; Effects of processing temperature on in-situ reinforcement formation in Al(Zn)/Al2O3(ZnO) nanocomposite. Metallurgical and Materials Engineering 2015, 21, 283-291, 10.30544/75.
- Jovid Rakhmonov; Giulio Timelli; Franco Bonollo; Characterization of the solidification path and microstructure of secondary Al-7Si-3Cu-0.3Mg alloy with Zr, V and Ni additions. Materials Characterization 2017, 128, 100-108, 10.1016/j.matchar.2017.03.039.
- M.R. Dehnavi; B. Niroumand; F. Ashrafizadeh; P.K. Rohatgi; Effects of continuous and discontinuous ultrasonic treatments on mechanical properties and microstructural characteristics of cast Al413–SiCnp nanocomposite. Materials Science and Engineering: A 2014, 617, 73-83, 10.1016/j.msea.2014.08.042.
- Rouholah Ashiri; Behzad Niroumand; F. Karimzadeh; Physical, mechanical and dry sliding wear properties of an Al–Si–Mg–Ni–Cu alloy under different processing conditions. Journal of Alloys and Compounds 2014, 582, 213-222, 10.1016/j.jallcom.2013.08.016.
- M. Reisi; B. Niroumand; Effects of stirring parameters on rheocast structure of Al–7.1wt.%Si alloy. Journal of Alloys and Compounds 2009, 470, 413-419, 10.1016/j.jallcom.2008.02.104.
- Mohammad Reza Nasresfahani; Behzad Niroumand; Ahmad Kermanpur; Mehdi Raeissi; Mohamad Reza Nasresfahani; EFFECTS OF APPLIED ELECTRIC CURRENT ON THE TIP RADIUS AND THE UNIVERSAL AMPLITUDE COEFFICIENT OF A SINGLE GROWING DENDRITE. Surface Review and Letters 2016, 23, 1550083, 10.1142/s0218625x15500833.
- Sung Su Jung; Yong Guk Son; Yong Ho Park; Young Cheol Lee; A Study on the Grain Refining Mechanisms and Melt Superheat Treatment of Aluminum-Bearing Mg Alloys. Metals 2022, 12, 464, 10.3390/met12030464.
- Yijiang Xu; Daniele Casari; Ragnvald Mathiesen; Yanjun Li; Revealing the heterogeneous nucleation behavior of equiaxed grains of inoculated Al alloys during directional solidification. Acta Materialia 2018, 149, 312-325, 10.1016/j.actamat.2018.02.058.
- Numerical Simulation of the Effects of a Phase Change Material (PCM) on Solidification Path of Gravity Sand Cast Al-Cu Alloy
- Noohi, Z. Tailoring Solidification Structure of Al-Cu Alloy Using Phase Change Materials (PCM); Isfahan University of Technology: Isfahan, Iran, 2022
- Fathi, M.; Isfahan University of Technology, Isfahan, Iran; Niroumand, B.; Isfahan University of Technology, Isfahan, Iran. Unpublished work. 2022
- Fathi, M. Targeted Control and In-Situ Observation of Solidification Structure of a Transparent Model Material Using Phase-Change Materials; Isfahan University of Technology: Isfahan, Iran, 2022.
