This rHeview provides general information on thrbal additives may have possible health benefits in
animals and humans of herbal additives, particularly thymol, whose phenolic group is responsible
for the neutralisation of free radicals, and information concerning its detection through body action,
bioavailability and mechanisms in rabbits. Plants containing thymol have been used in traditional
medicine for the treatment of various diseases, such as cardiovascular diseases, cancer and diabetes.
Although a great number of in vitro studies of cardiovascular and cancer diseases are available,
in vivo studies that confirm these findings have not been sufficiently reported. To determine the
beneficial dose, further clinical studies are necessary, with preclinical comprehensive research on
animal models.
- thymol
- biological activity
- health
- human
- animal
1. Introduction
2. Detection of Thymol and Its Metabolites in Humans and Animals
The pharmacodynamic activities of thyme extract or essential oil have been demonstrated in vitro. To confirm the beneficial effect of thymol found in vitro, its absorption, distribution, metabolism and excretion need to be detected in vivo [55][30]. Little is known about the bioactivity of thymol and its metabolites, as there are only few studies that have analysed thymol in body tissues (Table 1).
| Animal Species |
Applied Form | Detectable Compounds | Samples | References | |||
|---|---|---|---|---|---|---|---|
| human | thymol/orally | thymol glucuronide thymol sulphate thymohydroquinone sulphate thymol |
urine | [59] | [31] | ||
| human | Bronchipret | ® | TP/orally (equivalent to 1.08 mg thymol) |
thymol sulphate | plasma, urine | [55] | [30] |
| thymol glucuronide | urine | ||||||
| human | thymol/orally | p-cymene-2,5-diol p-cymene-2,3-diol p-cymene-3-ol-8-ene |
urine | [62] | [32] | ||
| human | dried thyme/orally | thymol sulphate caffeic acid sulphate hydroxyphenylpropionic acid sulphate |
plasma | [67] | [33] | ||
| thymol sulphate caffeic acid sulphate hydroxyphenylpropionic acid sulphate thymol glucuronide |
urine | ||||||
| rabbit | thymol/orally | glucuronic acid ethereal sulphuric acid thymol |
urine | [59] | [31] | ||
| rabbit | thymol/orally | thymol | plasma, small intestinal wall, liver, kidney, spleen, caecum, colon, muscle, faeces | [58] | [34] | ||
| rat | thymol/orally | p-cymene-2,5-diol p-cymene-3,9-diol p-cymene-3,7-diol thymol |
urine | [60] | [35] | ||
| rat | thyme extract/orally | thymol sulphate | plasma | [68] | [36] | ||
| laying hen | thyme extract/orally | p-cymene-2,3-diol thymol |
egg yolk | [61] | [37] | ||
| Japanese quail | thymol/orally | thymol | egg yolk, faeces | [74] | [38] | ||
| broiler chicken | dried | Thymi herba | /orally | thymol | plasma, small intestine, caecum, liver, muscle | [70] | [39] |
| broiler chicken | thyme essential oil/orally | thymol | plasma, liver, kidney, muscle, duodenal wall, gut content | [63] | [40] | ||
| broiler chicken | thyme essential oil/orally | thymol sulphate thymol glucuronide |
plasma, duodenal wall, liver | [76] | [41] | ||
| piglet | essential oil/orally (carvacrol, thymol, eugenol and trans-cinnamaldehyde) |
carvacrol thymol eugenol |
plasma | [64] | [42] | ||
| carvacrol thymol eugenol trans-cinnamaldehyde |
small intestine | ||||||
| carvacrol thymol eugenol trans-cinnamaldehyde |
bile | ||||||
| carvacrol thymol eugenol |
urine | ||||||
| piglet | Biomin | ® | P.E.P 1000 (main compounds thymol and carvacrol)/orally | thymol | plasma, kidney, faeces | [72] | [43] |
| piglet | Thymi herba | /orally | thymol | plasma | [73] | [44] | |
| bovine | Phyto-Mast (essential oil of | Thymus vulgaris | and oregano)/intramammary | thymol | milk, plasma, liver, kidney, fat | [65] | [45] |
| dairy cattle | Phyto-Mast (essential oil of | Thymus vulgaris | and oregano)/intramammary | thymol | milk, plasma, liver, kidney, fat | [66] | [46] |
| horse | Bronchipret (equivalent to 2–4 g thyme extract)/orally | thymol | plasma | [69] | [47] |
3. Bioavailability of Thymol Generally and in Rabbits as Model Animal
Phytogenic compounds and other foreign substances after oral administration are absorbed from the intestine, metabolised and eliminated from the organism. Once they reach the intestine or liver, they are converted during biotransformation processes (phase I and phase II) to more hydrophilic forms, and their pharmacological properties usually differ compared to the parental compound. It is also important to emphasise that metabolites can probably be deconjugated to the parental compound and express their pharmacological activity in this way. The major reactions occurring during phase I are oxidation, reduction and hydrolysis; phase II reactions are also called conjugation reactions and include glucuronidation, sulphation, acetylation, methylation, conjugation with glutathione and conjugation with amino acids [77,78,79,80,81,82][48][49][50][51][52][53].
The intestine plays an important role as a site for the absorption as well as biotransformation of thymol [63,76][40][41]. Thymol or its metabolites, after biotransformation processes in the intestinal wall, can be transported back into the intestinal lumen or are converted back to the parental compounds and redistributed within the organism through the systemic circulation [78][49]. Some part of the compounds are transported by the mesenteric vein into the liver, where they are metabolised, excreted into the duodenum in bile and again reabsorbed [57,78][49][54]. Bacova et al. [58][34] found 15% of thymol in the liver compared with the intestinal wall, which demonstrates the intensive absorption of thymol from the intestinal wall through the vena portae to the liver.
There are some barriers that the compounds must pass through during their metabolic path in the organism, and they limit their absorption [63,[40] 79][50]. The first-pass metabolism in the intestine decreases the number of molecules reaching the blood circulation, and then the first-pass metabolism in the liver represents another barrier for the distribution of compounds in the organism. In addition to this, many efflux transporters are bound mainly with lipophilic molecules, which are rapidly excreted from the organism, greatly limiting their bioavailability [58,78,80,81,82][34][49][51][52][53]. These biotransformation processes are probably the reason why only a trace amount of thymol is found in the muscle and fat tissues of broilers [70,77][39][48] and rabbits [58][34].
The rabbit’s digestive processes represent a complex system of the separation of digestible and indigestible parts of ingested food in the proximal colon [83][55]. The most important mechanism by which nutrients are released from ingested food is microbial fermentation in the caecum. The products of fermentation are either absorbed directly through the caecal wall or are re-ingested as caecotrophs [84][56]. The caecum and colon are the most important parts of a rabbit’s digestive system in connection with the original feature of digestion, caecotrophy.
Studying thymol bioavailability in different tissues is essential to understand its mechanism of action in target organs in which it can exert its biological role. Oral bioavailability represents the fraction of administered thymol reaching the systemic circulation and is a key parameter that affects its efficacy. Therefore, to propose an appropriate dose, the study of its oral bioavailability has received significant attention. However, only a few studies on thymol oral bioavailability have been carried out to date. The organ bioavailability barriers to thymol are depicted in Figure 1.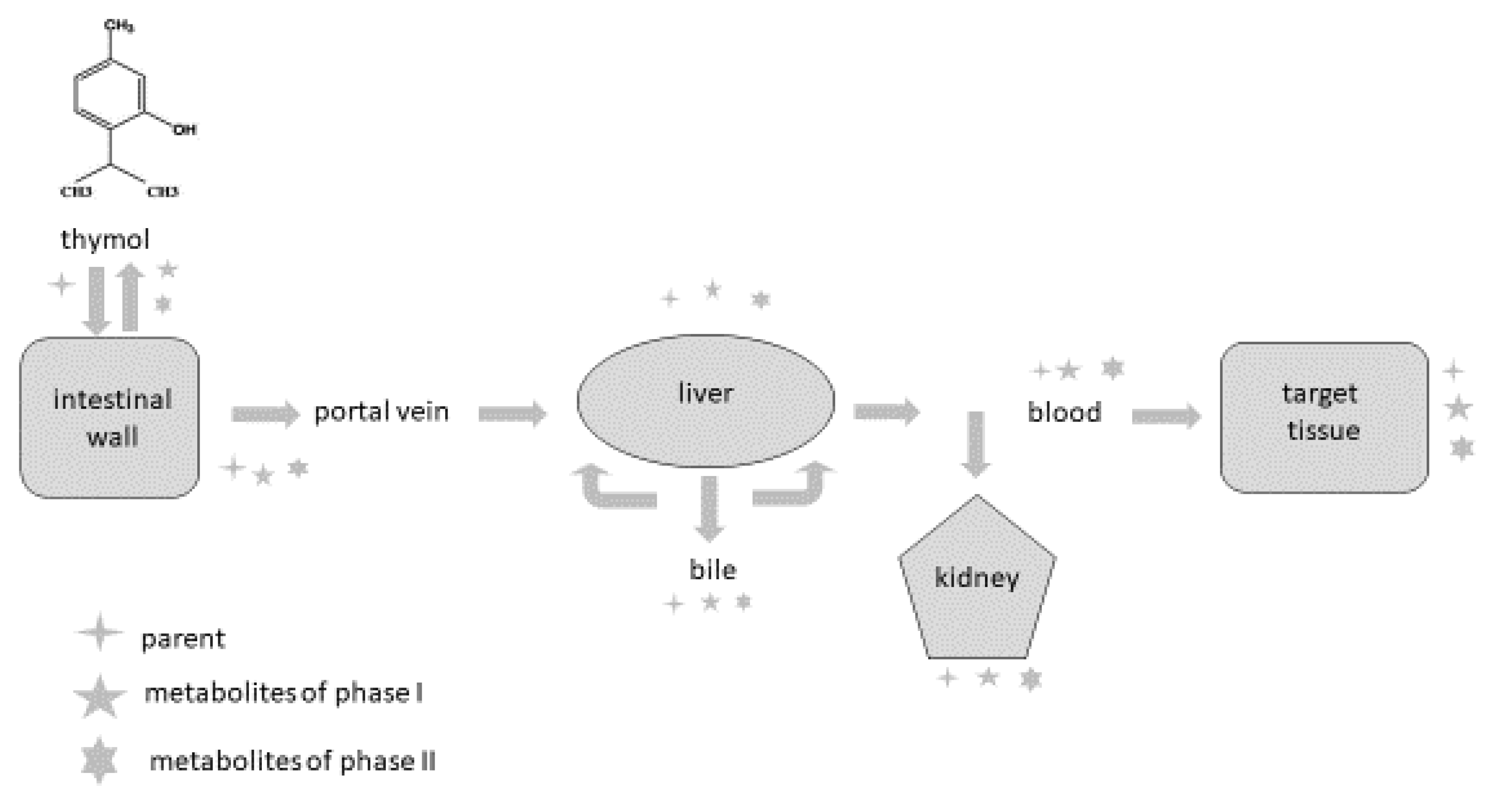
4. Conclusions
The presented data represent the available scientific information regarding the urgent need for more studies to precisely understand the metabolic processes and biological activity of thymol and its metabolites within organisms. This information will be useful for researchers, drug and pharmaceutical industries and the medical and veterinary sectors.
References
- Giacometti, J.; Kovacevic, D.B.; Putnik, P.; Gabric, D.; Bilusic, T.; Kresic, G.; Stulic, V.; Barba, F.J.; Chemat, F.; Barbosa-Canovas, G.; et al. Extraction of bioactive compounds and essential oils from Mediterranesn herbs by conventional and green innovative techniques: A review. Food. Res. Int. 2018, 113, 245–262.
- Oliveira, M.S.; Almeida, M.M.; Salazar, M.L.A.R.; Pires, F.C.S.; Bezzera, F.W.F.; Cunha, V.M.B.; Cordeiro, R.M.; Urbina, G.R.O.; Silva, A.P.S.; Pinto, R.H.H.; et al. Potential of medicinal use of essential oils from aromatic plants. In Potential of Essential Oils; El-Shemy, H., Ed.; IntechOpen: London, UK, 2018; pp. 1–19.
- Schmidt, B.; Ribnicky, D.M.; Poulev, A.; Logendra, S.; Cefalu, W.T.; Raskin, I. A natural history of botanical therapeutics. Metab. Clin. Exp. 2008, 57 (Suppl. S1), S3–S9.
- Giannenas, I.; Sidiropoulou, E.; Bonos, E.; Christaki, E.; Florou-Paneri, P. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives. In Feed Additives, Aromatic Plants and Herbs in Animal Nutrition and Health; Florou-Paneri, P., Christaki, E., Giannenas, I., Eds.; Elsevier Academic Press, Inc.: London, UK, 2020; pp. 1–15.
- Genser, D. Food and drug Interaction: Consequences for the nutrition/health status. Ann. Nutr. Metab. 2008, 52 (Suppl. S1), 29–32.
- Ribeiro dos Santos, R.; Andrade, M.; Sanches-Silva, A.; De Melo, N.R. Essential oils for food application: Natural substances with established biological activities. Food Bioprocess. Technol. 2017, 11, 43–71.
- Brown, E.D.; Wright, G.D. Antibacterial drugs discovery in the resistance era. Nature 2016, 529, 336–343.
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614.
- Tortorella, E.; Tedesco, P.; Esposito, P.F.; January, G.G.; Fani, R.; Jaspars, M.; de Pascale, D. Antibiotics from deep-sea microorganisms: Current discoveries and perspectives. Mar. Drugs 2018, 16, 355.
- Penesyan, A.; Khelleberg, S.; Egan, S. Development of novel drugs from marine surface associated microorganisms. Mar. Drugs 2010, 8, 438–459.
- Chavan, S.S.; Damale, M.G.; Devanand, B.S.; Sangshetti, J.N. Antibacterial and antifungal drugs from natural source: A review of clinical development. Nat. Prod. Clin. Trials 2018, 1, 114–164.
- Boucher, H.W.; Ambrose, P.G.; Chambers, H.F.; Ebright, R.H.; Jezek, A.; Murray, B.E.; Newland, J.G.; Ostrowsky, B.; Rex, J.H. White paper: Developing antimicrobial drugs for resistane pathogens, narrow-spectrum indivations, and unmet needs. J. Infect. Dis. 2017, 216, 228–236.
- Chen, S.; Song, J.; Sun, C.; Xu, J.; Zhu, Y.; Verpoorte, R.; Fan, T.P. Herbal genomics: Examining the biology of traditional medicines. Science 2015, 347, S27–S29.
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661.
- Anand, U.; Jacobo-Herrera, N.; Altemimi, A.; Lakhssassi, N. A comprehensive review on medicicnal plants as antimicrobial therapeutics: Potential avenues of biocompatible grug discovery. Metabolites 2019, 9, 258.
- Raskin, I.; Ribnicky, D.M.; Komarnytsky, S.; Ilic, N.; Poulev, A.; Borisjuk, N.; Brinker, A.; Moreno, D.A.; Ripoll, C.; Yakoby, N.; et al. Plants and human health in the twenty-first century. Trends Biotechnol. 2002, 20, 522–531.
- Castillo-Espaňa, P.; Cisneros-Estrada, A.; Garduno-Ramirez, M.L.; Hernandez-Abreu, O.; Ramirez, R.; Estrada-Soto, S. Preliminary ethnopharmacological survey of plants used in Mexico for the treatment of hypertension. Phcog. Rev. 2009, 3, 41–65.
- Giordani, R.; Hadef, Y.; Kaloustian, J. Compositions and antifungal activities of essential oils of some Algerian aromatic plants. Fitoterapia 2008, 79, 199–203.
- Al-Bayati, F.A. Synergistic antibacterial activity between Thymus vulgaris and Pimpinella anisum essential oils and methanol extracts. J. Ethnophamacol. 2008, 116, 403–406.
- Nikolić, M.; Glamočlija, J.; Ferreira, I.C.F.R.; Calheha, R.; Fernandes, A.; Markovič, T.; Markovič, D.; Giweli, A.; Sokovič, M. Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Cros. Prod. 2014, 52, 183–190.
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J.L.; Phil, D. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341.
- Chang, X.; Zhang, T.; Zhang, W.; Zhao, Z.; Sun, J. Natural drugs a treatment strategy for cardiovascular disease through the regulation of oxidative stress. Oxid. Med. Cell. Longev. 2020, 2020, 5430407.
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrient 2019, 11, 2090.
- Costa, G.; Fortuna, A.; Gonçalves, D.; Figueiredo, I.V.; Falcão, A.; Batista, M.T. Pharmacokinetics of Cymbopogon citratus infusion in rats after single oral dose administration. SOJ Pharm. Pharm. Sci. 2017, 4, 1–9.
- Yu, Y.M.; Chao, T.Y.; Chang, W.C.; Chang, M.J.; Lee, M.F. Thymol reduces oxidative stress, aortic intimal thickening, and inflammation-related gene expression in hyperlipidemic rabbits. J. Food Drug Anal. 2016, 24, 556–563.
- Suen, J.; Thomas, J.; Kranz, A.; Vun, S.; Miller, M. Effect of flavonoids on oxidative stress and inflammation in adults at risk of cardiovascular disease: A systematic review. Healthcare 2016, 4, 69.
- Al-Rawi, N.H.; Shahid, A.M. Oxidative stress, antioxidants, and lipid profile in the serum and saliva of individuals with coronary heart disease: Is there a link with periodontal health? Minerva Stomatol. 2017, 66, 212–225.
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722.
- Harrison, D.; Griendling, K.K.; Landmesser, U.; Hornig, B.; Drexler, H. Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 2003, 91, 7A–11A.
- Kohlert, C.; Schindler, G.; März, R.W.; Abel, G.; Brinkhaus, B.; Derendorf, H.; Gräfe, E.U.; Veit, M. Systemic availability and pharmacokinetics of thymol in humans. J. Clin. Pharmacol. 2002, 42, 731–737.
- Takada, M.; Agata, I.; Sakamoto, M.; Yagi, N.; Hayashi, N. On the metabolic detoxication of thymol in rabbit and man. J. Toxicol. Sci. 1979, 4, 341–350.
- Thalhamer, B.; Buchberger, W.; Waser, M. Identification of thymol phase I metabolites in human urine by headspace sorptive extraction combined with thermal desorption and gas chromatography mass spectrometry. J. Pharm. Biomed. Anal. 2011, 56, 64–69.
- Rubió, L.; Farràs, M.; de la Torre, R.; Macià, A.; Romero, M.P.; Valls, R.M.; Solà, R.; Farré, M.; Fitó, M.; Motilva, M.J. Metabolite profiling of olive oil and thyme phenols after a sustained intake of two phenol-enriched olive oils by humans: Identification of compliance markers. Food Res. Int. 2014, 65, 59–68.
- Bacova, K.; Zitterl Eglseer, K.; Karas Räuber, G.; Chrastinova, L.; Laukova, A.; Takacsova, M.; Pogany Simonova, M.; Placha, I. Effect of sustained administration of thymol on its bioaccessibility and bioavailability in rabbits. Animals 2021, 11, 2595.
- Austgulen, L.; Solheim, E.; Scheline, R. Metabolism in rats of p-cymene derivatives: Carvacrol and thymol. Pharmacol. Toxicol. 1987, 61, 98–102.
- Rubió, L.; Serra, A.; Chen, C.Y.O.; Macià, A.; Romero, M.P.; Covas, M.I.; Solà, R.; Motilva, M.J. Effects of co-occurring components from olive oil and thyme extracts on antioxidant status and their bioavailability in acute ingestion in rats. Food Funct. 2014, 5, 740–747.
- Krause, E.L.; Ternes, W. Bioavailability of the antioxidative thyme compounds thymol and p-cymene-2,3-diol in egg. Eur. Food Res. Technol. 1999, 209, 140–144.
- Fernandez, M.E.; Palacio, M.A.; Labaque, M.C. Thymol detection by solid-phase microextraction in faeces and egg yolk of Japanese quail. J. Chromatogr. B 2017, 1044, 39–46.
- Haselmeyer, A.; Zentek, J.; Chizzola, R. Effects of thyme as a feed additive in broiler chickens on thymol in gut contents, blood plasma, liver and muscle. J. Sci. Food Agric. 2015, 95, 504–508.
- Oceľová, V. Plant additives in relation to the animal gastrointestinal tract and metabolism of their main compounds. Master’s Thesis, Institute of Animal Physiology, Slovak Academy of Sciences, Košice, Slovakia, 2017.
- Pisarčíková, J.; Oceľová, V.; Faix, Š.; Plachá, I.; Calderón, A.I. Identification and quantification of thymol metabolites in plasma, liver and duodenal wall of broiler chickens using UHPLC-ESI-QTOF-MS. Biomed. Chromatogr. 2017, 31, e3881.
- Michiels, J.; Missotten, J.; Dierick, N.; Fremaut, D.; Maene, P.; De Smet, S. In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets. J. Sci. Food Agric. 2008, 88, 2371–2381.
- Zitterl-Eglseer, K.; Wetscherek, W.; Stoni, A.; Kroismayr, A.; Windisch, W. Bioavailability of essential oils of a phytobiotic feed additive and impact of performance and nutrient digestibility in weaned piglets. Bodenkult. J. Land Manag. Food Environ. 2008, 59, 121–129.
- Hagmüller, W.; Jugl-Chizzola, M.; Zitterl-Eglseer, K.; Gabler, C.; Spergser, J.; Chizzola, R.; Franz, C. The use of Thymi Herba as feed additive (0.1%, 0.5%, 1.0%) in weanling piglets with assessment of the shedding of haemolysing E. coli and the detection of thymol in the blood plasma. Berl. Münch. Tierärztl. Wochenschr. 2005, 119, 50–54.
- Armorini, S.; Yeatts, J.E.; Mullen, K.A.E.; Mason, S.E.; Mehmeti, E.; Anderson, K.L.; Washburn, S.P.; Baynes, R.E. Development of a HS-SPME-GC-MS/MS method for the quantitation of thymol and carvacrol in bovine matrices and to determine residue depletion in milk and tissues. J. Agric. Food Chem. 2016, 64, 7856–7865.
- Mason, S.E.; Mullen, K.A.E.; Anderson, K.L.; Washburn, S.P.; Yeatts, J.L.; Baynes, R.E. Pharmacokinetic analysis of thymol, carvacrol and diallyl disulfide after intramammary and topical applications in healthy organic dairy cattle. Food Addit. Contam. Part A 2017, 34, 740–749.
- Van den Hoven, R.; Zappe, H.; Zitterl-Eglseer, K.; Jugl, M.; Franz, C. Study of the effect of Bronchipret on the lung function of five Austrian saddle horses suffering recurrent airway obstruction (heaves). Vet. Rec. 2003, 152, 555–557.
- Oceľová, V.; Chizzola, R.; Pisarčíková, J.; Novak, J.; Ivanišinová, O.; Faix, Š. Effect of thyme essential oil supplementation on thymol content in blood plasma, liver, kidney and muscle in broiler chickens. Nat. Prod. Commun. 2016, 11, 1545–1550.
- Singh, R.; Hu, M. Drug metabolism in gastroinestinal tract. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Li, X., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 91–109.
- Hu, M.; Li, X. Barriers to oral bioavailability-an overview. In Oral Bioavailability: Basic Principles, Advanced Concepts, and Applications; Hu, M., Li, X., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 1–5.
- Parkinson, A. Biotransformation of Xenobiotics. In Cassaret & Doull’s Toxicology: The Basic Science of Poisons, 6th ed.; Klaassen, C.D., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2001; pp. 133–224.
- Rozman, K.K.; Klaassen, C.D. Absorption, distribution, and excretion of toxicants. In Cassaret & Doull’s Toxicology: The Basic Science of Poisons, 6th ed.; Klaassen, C.D., Ed.; The McGraw-Hill Companies: New York, NY, USA, 2001; pp. 107–132.
- Timbrell, J.A. Factors Affecting Toxic Responses: Disposition. Principles of Biochemical Toxicology, 4th ed.; Informa Healthcare USA, Inc.: New York, NY, USA, 2009; pp. 35–74.
- Bacova, K.; Zitterl-Eglseer, K.; Chrastinova, L.; Laukova, A.; Madarova, M.; Gancarcikova, S.; Sopkova, D.; Andrejcakova, Z.; Placha, I. Effect of thymol addition and withdrawal on some blood parameters, antioxidative defence system and fatty acid profile in rabbit muscle. Animals 2020, 10, 1248.
- Davies, R.R.; Davies, J.A.E.R. Rabbit gastrointestinal physiology. Vet. Clin. North Am. Exot. Anim. Pract. 2003, 6, 139–153.
- Campbell-Ward, M.L. Gastrointestinal physiology and nutrition. In Ferrets, Rabbits, and Rodents: Clinical Medicine and Surgery, 3rd ed.; Quesenberry, K.E., Carpenter, J.W., Eds.; Elsevier: Saint-Louis, MI, USA, 2012; pp. 183–192.
