Antioxidants are compounds that normally prevent lipid and protein oxidation. They play a major role in preventing many adverse conditions in the human body, including inflammation and cancer. Synthetic antioxidants are widely used in the food industry to prevent the production of adverse compounds that harm humans. However, plant and animal-based antioxidants are more appealing to consumers than synthetic antioxidants. Plant-based antioxidants are mainly phenolic compounds, carotenoids, and vitamins, while animal-based antioxidants are mainly whole protein or the peptides of meat, fish, egg, milk, and plant proteins. Plant-based antioxidants mainly consist of aromatic rings, while animal-based antioxidants mainly consist of amino acids. The phenolic compounds and peptides act differently in preventing oxidation and can use in the food and pharmaceutical industries. Therefore, compared with the animal-based antioxidants, plant-based compounds are more practical in the food industry. Even though plant base antioxidant compounds are good sources of antioxidants, animal-based peptides (individual peptides) cannot be considered antioxidants compounds to add to food. However, they can consider an ingredient that will enhance the antioxidant capacity. This review mainly compares plant and animal-based antioxidants' structure, efficacy, mechanisms, and applications.
1. Introduction
Plants contain many natural compounds that have antioxidant activity. These compounds can be categorized into vitamins (Vitamin C and E), polyphenols (flavonoids, phenolic acids, stilbenes, lignans), and terpenoid groups. Fruits and vegetables are rich sources of vitamin C and E. Among the fruits are the family Rosaceae (sour cherry, strawberry, blackberry), Empetraceae (cowberry), Ticaceae (blueberry), Asteraceae (sunflower seed), and Punicaceae (pomegranate), which are rich sources of these vitamins. Broccoli, brussels sprouts, green cabbage, tomatoes, cauliflowers, lettuce, and leeks are vegetable groups with high vitamin C and E. Vitamins in plants act as primary antioxidant substances
[1][15]: Vitamin E acts as an essential lipid-soluble antioxidant, while vitamin C protects against oxidative stress-induced cellular damages
[2][3][16,17]. Both vitamin E and vitamin C are used as antioxidants in foods, but the effect of vitamin C is marginal.
Polyphenols are the major plant antioxidants with various structural and functional characteristics and biological properties
[4][5][6][11,18,19]. Phenolic compounds synthesize from phenylalanine or tyrosine through the shikimic acid pathway. They can vary from simple compounds to conjugated complex substances. These compounds vary from 500 to 4000 Da molecular weight, and over 12 phenolic hydroxyl groups are found among the phenolic compounds
[7][8][4,20]. They can be subcategorized into phenolic acids, flavonoids, stilbenes, and lignans
[9][10][6,21]. They are found in plant foods such as fruits, cereals, seeds, berries, and plant-based products such as wine, tea, and vegetable oils
[11][12][22,23].
2. Phenolic Acids
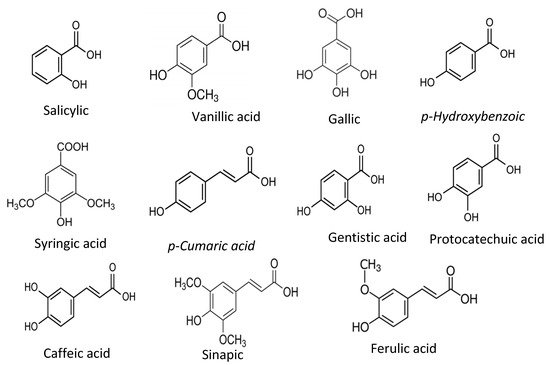
Phenolic acids are the derivatives of benzoic acids and cinnamic acids, and salicylic acid, gentisic acid, p-Hydoxybenzoic acid, protocatechuic acid, vanillic acid, syringic acid, gallic acid, p-coumaric acid, ferulic acid, caffeic acid, and sinapic acid are among the most common phenolic compounds present in the food plants and common phenolic acid compounds illustrated in Figure 12.
Figure 12. Common phenolic acids found in food plants.
The antioxidant activities of those phenolic acids are confirmed by several antioxidant assays, including the DPPH radical scavenging assay, ABTS radical scavenging activity, and β-carotene assay
[13][5]. The radical scavenging activities of these phenolic acids mainly depend on the hydroxy moieties attached to the phenyl rings of benzoic and cinnamic acids
[14][15][24,25].
3. Flavonoids
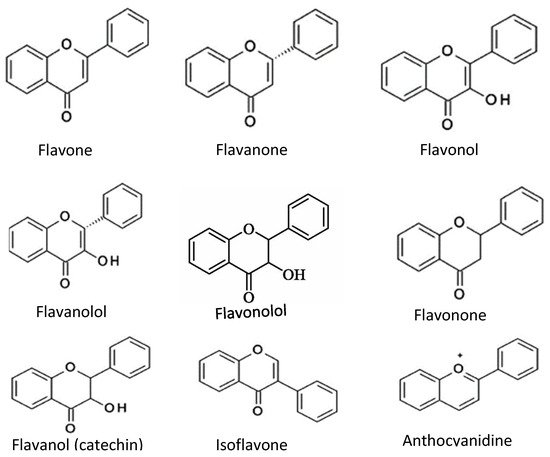
Flavonoids consist of two outer aromatic rings with three carbon rings. These flavonoids include flavone, flavanol, flavanone, flavanonol, flavonone, flavononol, flavanol (catechin), isoflavone, and anthocyanidin (
Figure 23)
[9][6]. The derivatives of the flavonoids have inhibitory properties against acetylcholinesterase
[16][26].
Figure 23. Chemical structures of common flavonoids.
4. Stilbenes
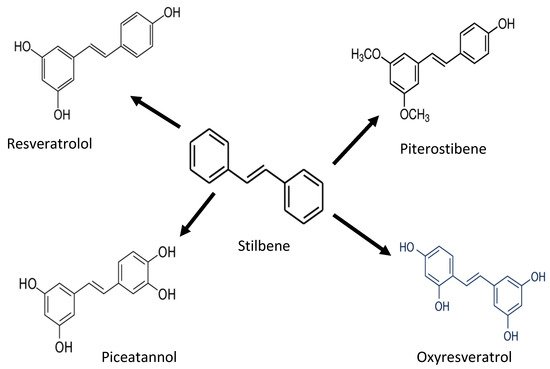
Stilbenoides/stilbenes are another phenolic compound commonly found in berries, grapevines, and peanuts. Stilbenoids are hydroxylated stilbene derivatives (i.e., resveratrol). The most common stilbenes are piceid, resveratrol, piceatannol, and pterostilbene (
Figure 34). However, only resveratrol has antioxidant potential against proteins and lipids
[17][18][19][27,28,29]. Endogenous compounds such as superoxide dismutase (SOD), catalase, and glutathione neutralize oxidative stress induced by UV radiation. Stilbenes, such as resveratrol, can increase the activity of antioxidant enzymes (glutathione
S-transferase) and increase the SOD level. In addition, pterostilbenes reduce oxidative damage by activating the endogenous antioxidant enzymes
[19][29].
Figure 34. Chemical structure of stilbene and its derivatives.
5. Lignans
Lignans are precursors to phytoestrogens and synthesized from phenylalanine with dimerization of substituted cinnamic alcohols. Sesame seeds and flax seeds are the main sources of lignans. Secoisolariciresinol, matairesinol, pinoresinol, and lariciresinol are the common lignans from flax seeds and sesamin, sesamoiln, sesamolinol, and sesaminol are the common lignans from sesame seeds
[20][30]. Sesamin and sesamolin possess antioxidant, neuroprotective, and anticancer activities, but sesamol, their decomposition product during the roasting process of sesame seeds, is the major antioxidant of the sesame seeds
[21][22][23][24][31,32,33,34]. The common plant-based phenolic antioxidants are summarized in
Table 1.
Table 1.
Most common plant-based phenolic antioxidants and their potential applications.
6. Terpenes and Terpenoids
Terpenes and terpenoids are also good antioxidants from plant sources. They are the largest secondary metabolites of plants. Terpenes and terpenoids contain a hydrocarbon skeleton with five carbons (isoprene), and two or more isoprene molecules polymerize and form various terpenes. Most of them are non-polar compounds
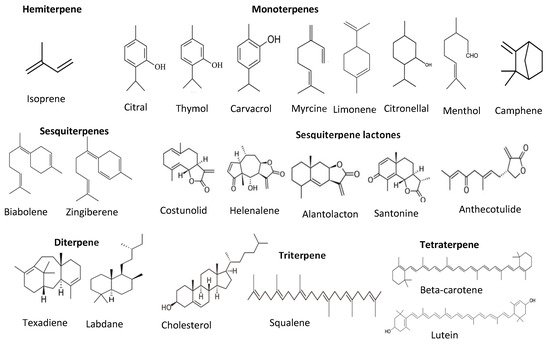
[35][45]. Plant oils such as pine oil, vegetables such as carrots, and some fruits such as lemon and orange are rich sources of terpenes and terpenoids. These compounds can further classify into monoterpenes (C-10), sesquiterpenes (C-15), diterpenes (C-20), triterpenes (C-30), tetraterpenes (C-40), or carotenoids, polyterpenes, norisopernoids, and sesquatreterpenes (
Figure 46). These compounds have antioxidant and antimicrobial activities, contributing odor and flavor and other health-promoting properties such as relieving stress and depression, reducing depression and migraines, and antiaging and anticancer properties
[25][36][35,46].
Figure 46. Structure of different classes of terpenoids.
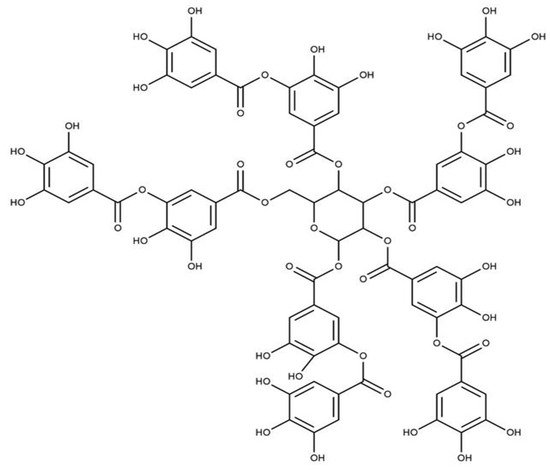
7. Tannins
Tannins are another group of phenolic antioxidants in plants and can be divided into two main sub-classes: condensed tannins and hydrolyzable tannins. The condensed tannins are biopolymers based on flavan-3-ols, and gallic and ellagic acid derivatives (gallotannins and ellagitannins) are the main components with antioxidant properties
[26][29][36,39]. Gallotannins are natural polymers formed by the esterification of D-glucose and gallic acid hydroxyl groups. Proanthocyanidins can donate hydrogen atoms/electrons and act as an antioxidant compound. Proanthocyanidins are abundant in green tea and bearberry
[14][28][24,38]. Tannin extracts from red beans, adzuki beans, lentils, fava beans, and broad beans showed better antioxidant activity than the flavonoids and phenolic acids separated from the same plant materials
[27][30][31][37,40,41]. The chemical structure of the tannic acid is shown in
Figure 57.
Figure 57. Structure of Tannic acid.
8. Sources of Plant Antioxidants
Oilseeds, cereal grains, legumes, tea, coffee, tree nuts, fruits, and berries are excellent sources of plant antioxidants. Among the oilseeds, rapeseed and canola seeds have very high levels of phenolic compounds
[14][37][24,47]. Sinapine (choline esters of sinapic acid) and sinapic acids are the main phenolic compounds in rapeseed
[38][48]. Most cereals contain phenolic compounds, and ferulic acid is the most dominant type. However, some wheat cultivars contain high levels of
p-coumaric, sinapic, and caffeic acids. Rice and rice bran contain γ-oryzanol, an ester of triterpene alcohols, and plant sterols
[32][42] with strong antioxidant properties. Legumes also contain high phenolic acid, flavonoids, and tannins. Catechin, epicatechin glucosides, procyanidin dimers, quercetin glucoside, and
p-coumaric are the main phenolic compounds in green lentils
[14][24]. The leaves and seeds of legumes are considered a good source of lignans. Lamiaceae is a good source of phenolic acid, rosmarininc and caffeic acid, flavonoids, hispidulin, nepetin, luteolin, and apigenin
[33][43]. Oregano (
Origanum vulgare L.) is another plant that belongs to the family and is rich in phenolic antioxidant compounds such as rosmarinic acid and chlorogenic acid and flavonoids such as hyperoside and isoquercitrin
[34][44].
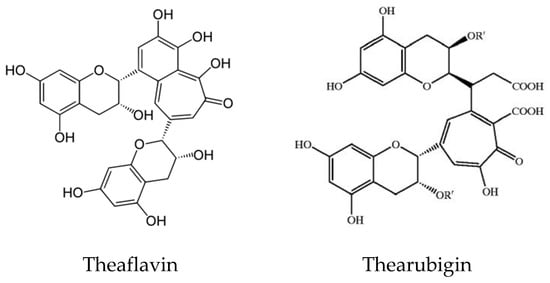
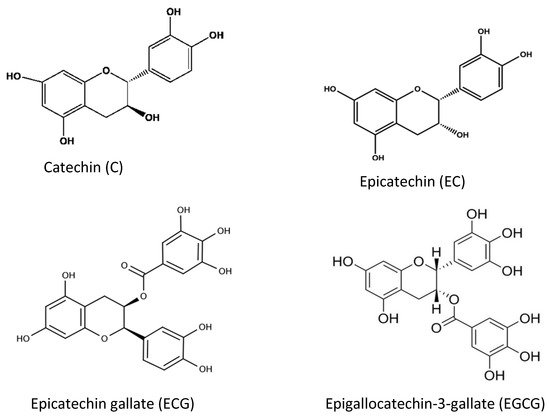
Tea and coffee are also rich sources of natural antioxidant compounds. Different antioxidants are found in green tea and black tea: theaflavin and thearubigin are the main antioxidant compounds in black tea (
Figure 68), while the isomers of catechins are the main active antioxidant compounds in green tea (
Figure 79). These are polyphenols, and the content varies among cultivars and by the method of processing
[39][40][41][49,50,51].
Figure 68. Structure of Theaflavin and Thearubigin in black tea.
Figure 79. Different chemical structures of catechins found in green tea.
Chlorogenic acids and their derivatives, including caffeoylquinic acids, caffeoylquinic acids, feruloyquinic acids,
p-coumaroylquinic acids, caffeic acids, and ferulic acids, are the predominant phenolic compounds in coffee beans
[42][52]. However, during heat processing, these phenolic compounds convert to quinolones and melanoidins
[43][53]. Tree nuts are also rich sources of phenolic compounds: catechin, epicatechin, epicatechin 3-gallate, and procyanidins are rich in hazelnut kernels
[44][54], and chlorogenic acid, caftaric acid, ferulic acid, gentisic acid, caffeic acid,
p-coumaric acid, sinapic acid, isoquercitrin, rutozid, myricetin, fisetin, quercitrin, quercetin, luteolin, kaempferol, patuletin, hyperoside, and apigenin are rich in walnuts
[45][55].
Fruits such as apples contain high amounts of polyphenols, hydroxycinnamates, flavonols, anthocyanins, and dihydrochalcones
[46][56]. The main antioxidant compounds in plums are gallic acid, rutin, resorcinol, chlorogenic acid, catechin, and ellagic acid
[47][8]. Berries, including strawberry, blackberry, blueberry, and cranberry, are rich in anthocyanins, flavonols, phenolic acids, and hydrolyzable tannins
[48][57].
Phenolic compounds are mainly extracted using subcritical water. The extraction of phenolic compounds is based on hot water treatments called brewing. The temperature of the water and soaking duration determine the yield of the extracted phenolic compounds
[43][49][50][53,58,59]. Solvent extraction is used to separate phenolic compounds from plant materials. Solvents such as methanol, acetone, ethanol, propanol, dimethylformamide, and ethyl acetate are mainly organic solvents. Different percentages of organic solvents separate the phenolic compounds
[37][47]. The solvent and sample extraction time and ratio determine the yield and the purity of the phenolic compounds separated
[51][52][60,61]. Furthermore, alkaline and acid hydrolysates separate these phenolic compounds
[53][62].
The separated phenolic compounds incorporated into edible oils rich in unsaturated fatty acids effectively prevented lipid oxidation
[54][55][56][57][58][59][60][61][63,64,65,66,67,68,69,70]. Combining the plant extracts rich in phenolic compounds, and synthetic antioxidants maintained the quality of many food products during storage
[62][71]. Phenolic compounds are also widely used as natural antioxidants in meat and meat products. Among the plant extracts, almond seeds, grape seeds, and rosemary extracts are widely used as a natural preservative in beef and pork-based products
[63][64][65][72,73,74]. Incorporating rosemary and green tea extracts into butter effectively prevented lipid oxidation
[66][75]. Other plant extracts are also added to prevent lipid oxidation in seafood-based products
[67][68][76,77].