Maize (Zea mays), also called corn, is one of the top three staple food crops worldwide and is also utilized as feed (e.g., feed grain and silage) and a source of biofuel (e.g., bioethanol). Maize production is hampered by a myriad of factors, including although not limited to fungal diseases, which reduce grain yield and downgrade kernel quality. One such disease is anthracnose leaf blight and stalk rot (ALB and ASR) caused by the hemibiotrophic fungal pathogen Colletotrichum graminicola. The pathogen deploys a biphasic infection strategy to colonize susceptible maize genotypes, comprising latent (symptomless) biotrophic and destructive (symptomatic) necrotrophic phases. However, the resistant maize genotypes restrict the C. graminicola infection and in planta fungal proliferation during the biotrophic phase of the infection. Some studies on the inheritance of ASR resistance in the populations derived from biparental resistant and susceptible genotypes reveal that anthracnose is likely a gene-for-gene disease in which the resistant maize genotypes and C. graminicola recognize each other by their matching pairs of nucleotide-binding leucine-rich repeat resistance (NLR) proteins (whose coding genes are localized in disease QTL) and effectors (1–2 effectors/NLR) during the biotrophic phase of infection. The Z. mays genome encodes approximately 144 NLRs, two of which, RCg1 and RCg1b, located on chromosome 4, were cloned and functionally validated for their role in ASR resistance. Here, we discuss the genetic architecture of anthracnose resistance in the resistant maize genotypes, i.e., disease QTL and underlying resistance genes. In addition, this review also highlights the disease cycle of C. graminicola and molecular factors (e.g., virulence/pathogenicity factors such as effectors and secondary metabolites) that contribute to the pathogen’s virulence on maize. A detailed understanding of molecular genetics underlying the maize–C. graminicola interaction will help devise effective management strategies against ALB and ASR.
- anthracnose leaf blight
- anthracnose stalk rot
- hemibiotrophic pathogens
- gene-for-gene diseases
- NLR immune receptors
- effectors
1. Introduction
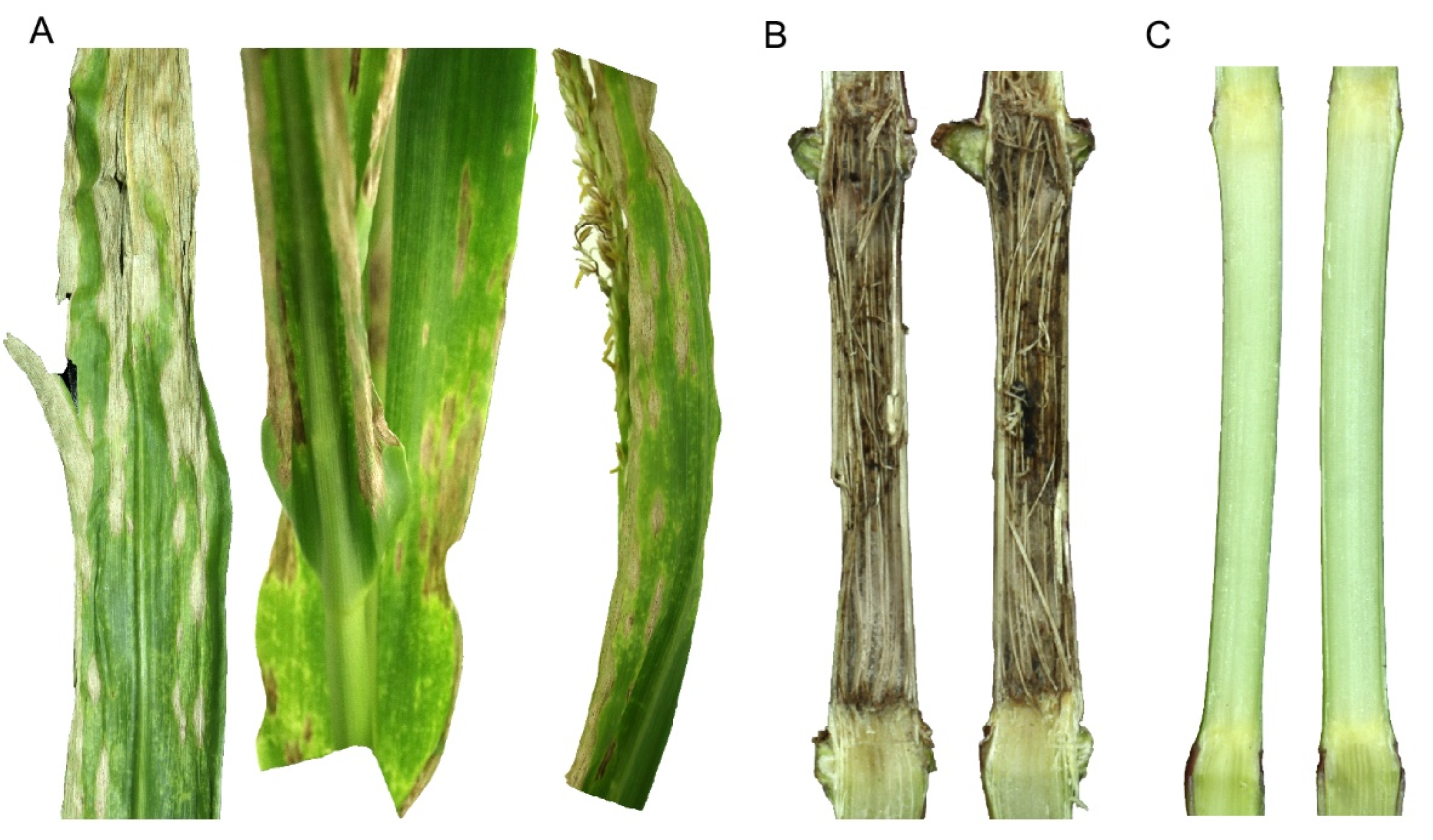

2. Genetic Architecture of Anthracnose Resistance in Maize
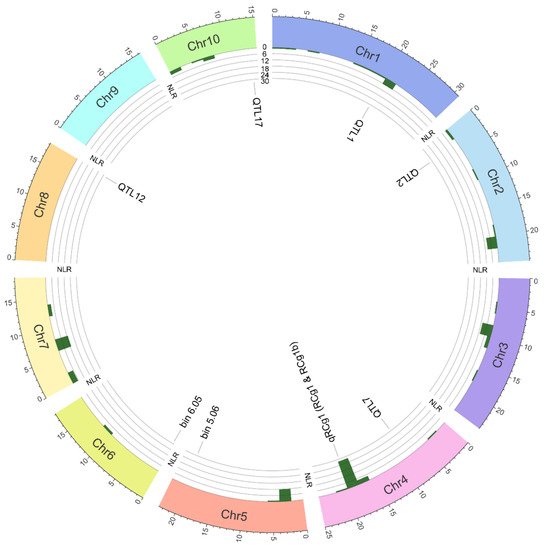
In the absence of an adaptative resistance, genetic resistance in plants relies on the detection of conserved and variable pathogen molecules called, respectively, pathogen-associated molecular patterns (PAMPs) and effectors. The archetypical PAMPs include chitin (a major constituent of the fungal cell wall) and flagellin (a principal constituent of bacterial flagella). Effectors are proteinaceous and non-proteinaceous molecules that pathogens secrete into hosts to manipulate host metabolism and/or to disarm the immune system, thereby facilitating their colonization and proliferation. To detect PAMPs and effectors, plants possess a two-branched multilayered immune system. The first branch functions at the extracellular level and is called the PAMP-triggered immune (PTI) system, whereas the second branch, called the effector-triggered immune (ETI) system, acts at the intracellular level. The PTI system is activated when its components, transmembrane pattern recognition receptors (PRRs, e.g., receptor-like kinases and receptor-like proteins) detect PAMPs in the apoplast. Once the pathogens are able to disable the PTI system using their effector arsenal successfully, the ETI system comes into play, whereby nucleotide-binding leucine-rich repeat resistance proteins (NLRs, a major component of the ETI system) recognize variable effectors—that undermine both PTI and ETI systems and promote pathogen colonization—either directly through physical interactions [12][18], or indirectly by monitoring the integrity of guarded host virulence targets (called guardees) [13][14][19,20] or mimics thereof (called decoys) [15][21] in the cytoplasm. Z. mays (2n = 20) is a diploid species, whose nuclear genome (2.13 Gb) encodes ~144 NLRs (Figure 3). The number of NLRs in maize is relatively lower than those reported for other cereal crops, e.g., Oryza sativa (438), Triticum aestivum (627) and Hordeum vulgare (224) [16][22].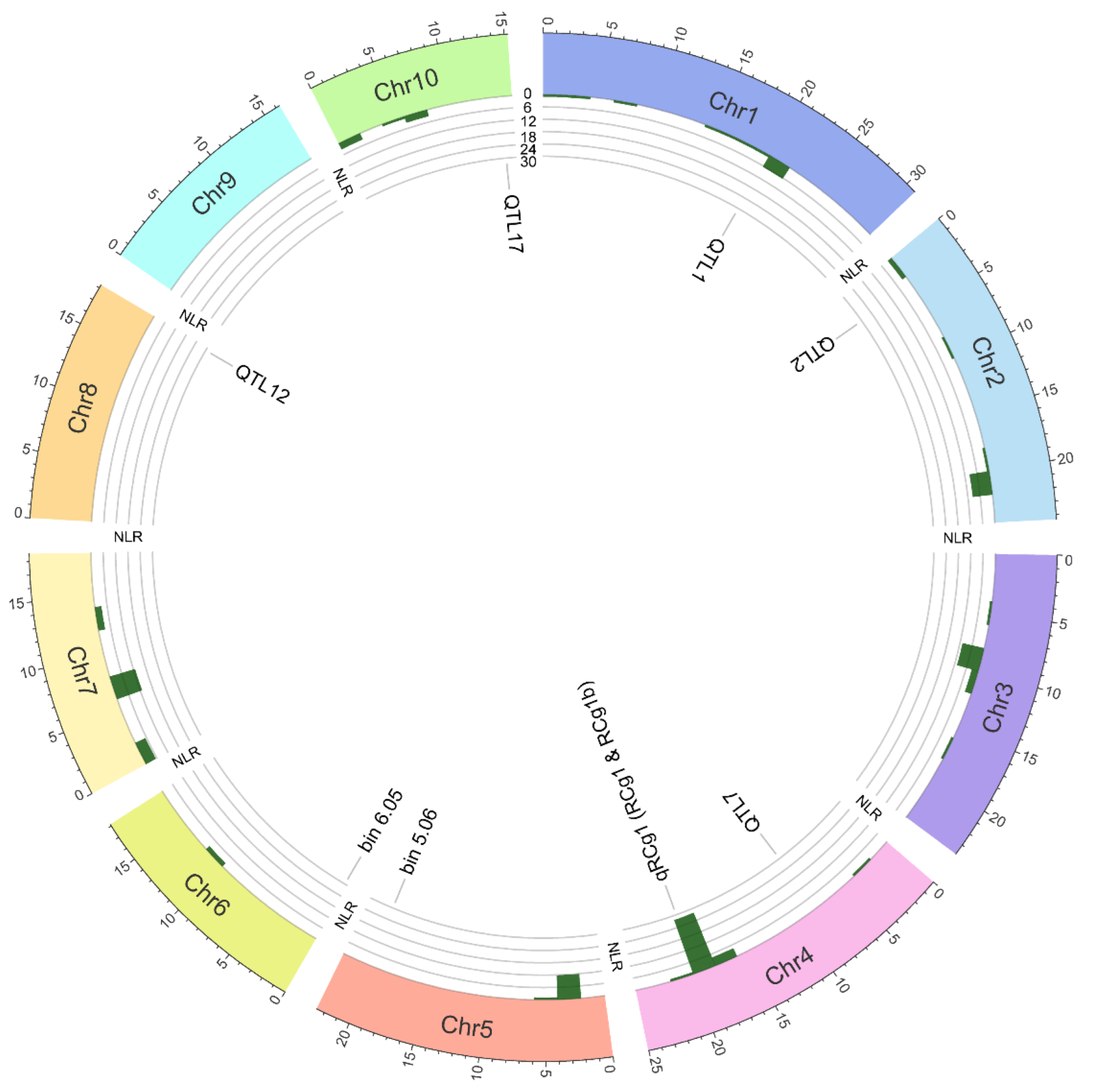
| Population | Resistance Source | Population | QTL | LG | Linked Markers | Marker Interval | ASR/ALB | Reference | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DE811ASR × DE811 | DE811ASR (MP305) | RIL | RCg1 | 4 | UMC66a-UMC15a | 397.4–525.8 cM | ASR | [26] | [32] | ||
| DE811ASR × LH132 | DE811ASR (MP305) | RIL | RCg1 | 4 | UMC66a-UMC15a | 397.4–525.8 cM | ASR | [26] | [32] | ||
| DE811ASR × DE811 | DE811ASR (MP305) | NIL | RCg1 | 4 | MZA2591-PHI093 | 61.0–63.0 cM | ASR | [27][28] | [34,36] | ||
| S11 × DK8883 | S11 | F | 6:7 | HIF | bin 5.06 | 5 | umc2216 | 518.4 cM | ASR | [29] | [33] |
| S11 | F | 6:7 | HIF | bin 6.05 | 6 | bngl2249 | 278.0 cM | ASR | [29] | [33] | |
| LB58 × A632 | LB58 | BC | CgL | - | - | ALB | [22] | [28] | |||
| LB31 × B37 | LB31 | RIL and BC | CgR | - | ASR | [23] | [29] | ||||
| L04-2 × L95-1 | L04-2 | RIL | QTL1 | 1 | E32M48_308-E42M50_174 | 177.9–189.4 cM | ALB | [21] | [27] | ||
| L04-2 | RIL | QTL2 | 2 | E35M56_680-E35M56_112 | 0.0–14.1 cM | ALB | |||||
| L04-2 | RIL | QTL3 | 3 | E42M51_162-E42M50_76 | 0.0–7.6 cM | ALB | |||||
| L04-2 | RIL | QTL4 | 3 | E32M48_167-E32M59_104 | 51.0–61.4 cM | ALB | |||||
| L04-2 | RIL | QTL5 | 4 | E35M60_87-E32M60_185 | 0.0–10.4 cM | ALB | |||||
| L04-2 | RIL | QTL6 | 4 | E32M52_73-E44M51_84 | 15.3–34.8 cM | ALB | |||||
| L04-2 | RIL | QTL7 | 4 | Umc1511-E32M53_434 | 88.1–119.3 cM | ALB | |||||
| L04-2 | RIL | QTL8 | 4 | E32M53_434-E44M51_135 | 119.3–137.7 cM | ALB | |||||
| L04-2 | RIL | QTL9 | 5 | E32M48_532-E32M50_139 | 242.0–244.3 cM | ALB | |||||
| L04-2 | RIL | QTL10 | 8 | E35M60_80-E32M50_100 | 0.0–23.9 cM | ALB | |||||
| L04-2 | RIL | QTL11 | 8 | E32M60_94-E32M50_248 | 57.3–74.1 cM | ALB | |||||
| L04-2 | RIL | QTL12 | 8 | E35M60_86-Phi015 | 85.5–107.5 cM | ALB | |||||
| L04-2 | RIL | QTL13S | 9 | E32M48_562-E32M48_97 | 126.4–157.7 cM | ALB | |||||
| L04-2 | RIL | QTL14 | 9 | E32M51_314-E35M56_174 | 179.1–201.1 cM | ALB | |||||
| L04-2 | RIL | QTL15S | 10 | E32M49_698-E32M59_207 | 28.3–58.7 cM | ALB | |||||
| L04-2 | RIL | QTL16S | 10 | E32M50_118-E44M56_81 | 85.4–109.1 cM | ALB | |||||
| L04-2 | RIL | QTL17S | 10 | E32M59_76-Umc1084 | 161.6–191.7 cM | ALB |
Funding: The research was financially supported by the National Natural Science Foundation of China (Grant No. 32172363) and the Chinese Universities Scientific Fund (Grant No. 10092004).
References
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A paramount staple crop in the context of global nutrition. Rev. Food Sci. Food Saf. 2010, 9, 417–436.
- Asseng, S.; Ewert, F.; Martre, P.; Rötter, R.P.; Lobell, D.B.; Cammarano, D.; Kimball, B.A.; Ottman, M.J.; Wall, G.W.; White, J.W.; et al. Rising temperatures reduce global wheat production. Clim. Chang. 2015, 5, 143–147.
- Foley, J. It’s Time to Rethink America’s Corn System. Scientific American. Available online: https://scientificamerican.com (accessed on).
- Yang, Q.; Balint-Kurti, P.; Xu, M. Quantitative disease resistance: Dissection and adoption in maize. Plant 2017, 10, 402–413.
- Mueller, D.S.; Wise, K.A.; Sisson, A.J.; Allen, T.W.; Bergstrom, G.C.; Bissonnette, K.M.; Bradley, C.A.; Byamukama, E.; Chilvers, M.I.; Collins, A.A.; et al. Corn yield loss estimates due to diseases in the United States and Ontario, Canada, from 2016 to 2019. Plant Health Prog. 2020, 21, 238–247.
- Ma, W.; Yang, J.; Gao, X.; Han, T.; Liu, J.; Ding, J.; Zhao, W.; Peng, Y.; Bhadauria, V. First report of Didymella glomerata causing Didymella leaf blight on maize in China. Plant Dis. 2022, volume, article number. https://org/10.1094/PDIS-02-22-0282-PDN.
- Mahuku, G. Maize pathology in Asia: Opportunities and challenges for breeding disease-resistant maize. Asian Reg. Maize Workshop 2010, 10, 361–366.
- Williams, L.E.; Willis, G.M. Disease of corn caused by Colletotrichum graminicola. Phytopathology 1963, 53, 364–365.
- White, D.G.; Yanney, J.; Natti, T.A. Anthracnose stalk rot. Annu. Corn Sorghum Res. Conf. 1979, 34, 1–15.
- Bergstrom, G.C.; Nicholson, R.L. The biology of corn anthracnose: Knowledge to exploit for improved management. Plant Dis. 1999, 83, 596–608.
- Frey, T.J.; Weldekidan T.; Colbert, T. ; Wolters, P.J.C.C.; Hawk, J.A. Fitness evaluation of Rcg1, a locus that confers resistance to Colletotrichum graminicola (Ces.) G.W. Wils. using near-isogenic maize hybrids. Crop Sci. 2011, 51, 1551–1563.
- Duan, C.X.; Guo, C.; Yang, Z.H.; Sun, S.L.; Zhu, Z.D.; Wang, X.M. First report of anthracnose leaf blight of maize caused by Colletotrichum graminicola in China. Plant Dis. 2019, 103, article number. https://org/10.1094/PDIS-12-18-2140-PDN.
- Robertson, A. An in-depth look at the Corn-Colletotrichum graminicola (causal organism of anthracnose) pathosystem. In Proceedings of the 25th Annual Integrated Crop Management Conference, Ames, IA, USA, 4–5 December 2013; Volume 18, pp. 87–89.
- Mims, C.W.; Vaillancourt, L.J. Ultrastructural characterization of infection and colonization of maize leaves by Colletotrichum graminicola, and by a graminicola pathogenicity mutant. Phytopathology 2002, 92, 803–812.
- Vizvary, M.A.; Warren, H.L. Survival of Colletotrichum graminicola in soil. Phytopathology 1982, 72, 522–525.
- Keller, N.P.; Bergstrom, G.C.; Carruthers, R.I. Potential yield reductions in maize associated with an anthracnose⁄European corn borer pest complex in New York. Phytopathology 1986, 76, 586–589.
- Callaway, M.B.; Smith, M.E.; Coffman, W.R. Effect of anthracnose stalk rot on grain yield and related traits of maize adapted to the northeastern United States. J. Plant Sci. 1992, 72, 1031–1036.
- Dodds, P.N.; Rathjen, J.P. Plant immunity: Towards an integrated view of plant-pathogen interactions. Rev. Genet. 2010, 11, 539–548.
- van der Biezen, E.A.; Jones, J.D.G. Plant disease-resistance proteins and the gene-for-gene concept. Trends Biochem. Sci. 1998, 23, 454–456.
- Dangl, J.L.; Jones, J.D.G. Plant pathogens and integrated defence responses to infection. Nature 2001, 411, 826–833.
- van der Hoorn, R.A.; Kamoun, S. From Guard to Decoy: A new model for perception of plant pathogen effectors. Plant Cell 2008, 20, 2009–2017.
- Sarris, P.F.; Cevik, V.; Dagdas, G.; Jones, J.D.G.; Krasileva, K.V. Comparative analysis of plant immune receptor architectures uncovers host proteins likely targeted by pathogens. BMC Biol. 2016, 14, e8.
- Tsuda, K.; Katagiri, F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Opin. Plant Biol. 2010, 13, 459–465.
- Jones, D.G.J.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329.
- Lim, S.M.; White, D.G. Estimates of heterosis and combining ability for resistance of maize to Colletotrichum graminicola. Phytopathology 1978, 68, 1336–1342.
- Carson, M.L.; Hooker, A.L. Reciprocal translocation testcross analysis of genes for anthracnose stalk rot resistance in a corn inbred line. Phytopathology 1982, 72, 175–177.
- Romanek, C.; Matiello, R.R.; Coelho, C.D.J.; Schafascheck, L.; Silva, D.F.G.; Gardingo, J.R. QTL mapping to anthracnose leaf blight resistance in tropical maize. Crop Breed. Appl. Biotechnol. 2017, 17, 390–398.
- Badu-Apraku, B.; Gracen, V.E.; Bergstrom, G.C. A major gene for resistance to anthracnose leaf blight in maize. Plant Breed. 1987, 98, 194–199.
- Badu-Apraku, B.; Gracen, V.E.; Bergstrom, G.C. A major gene for resistance to stalk rot in maize. Phytopathology 1987, 77, 957–959.
- Carson, M.L. Sources and Inheritance of Resistance to Anthracnose Stalk Rot of Corn. Ph.D. Thesis, University of Illinois, Urbana-Champaign, IL, USA, 1981.
- Toman, J.R.; White, D.J. Inheritance of resistance to stalk rot of corn. Phytopathology 1993, 83, 981–986.
- Jung, M.; Weldekidan, T.; Schaff, D.; Paterson, A.; Tingey, S.; Hawk, J. Generation means analysis and quantitative trait locus mapping of anthracnose stalk rot genes in maize. Appl. Genet. 1994, 89, 413–418.
- Chung, C.L.; Poland, J.; Kump, K.; Benson, J.; Longfellow, J.; Walsh, E.; Balint-Kurti, P.; Nelson, R. Targeted discovery of quantitative trait loci for resistance to northern leaf blight and other diseases of maize. Appl. Genet. 2011, 123, 307–326.
- Broglie, K.E.; Butler, K.H.; Butruille, M.G.; da Silva, C.A.; Frey, T.J.; Hawk, J.A.; Jaqueth, J.S.; Jones, E.S.; Multani, D.S.; Wolters, P.J.C.C. 2006. Polynucleotides and methods for making plants resistant to fungal pathogens. U.S. Patent 7 619 133, 17 November 2009.
- Frey, T.J. Fine Mapping, Cloning, Verification, and Fitness Evaluation of a QTL, Rcg1, which Confers Resistance to Colletotrichum graminicola in Maize. Ph.D. Thesis, University of Delaware, Newark, DE, USA, 2006
- Broglie, K.E.; Butler, K.H. Polynucleotides and Methods for Making Plants Resistant to Fungal Pathogens. U.S. Patent Application 20090307798, 2009.
- Hogenhout, S.A.; van der Hoorn, R.A.L.; Terauchi, R.; Kamoun, S. 2009. Emerging concepts in effector biology of plant-associated organisms. Plant Microbe Interact. 2009, 22, 115–122.
- O’Connell, R.J.; Thon, M.R.; Hacquard, S.; Amyotte, S.G.; Kleemann, J.; Torres, M.F.; Damm, U.; Buiate, E.A.; Epstein, L.; Alkan, N.; et al. Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Genet. 2012, 44, 1060–1065.
- Vargas, W.A.; Martín, J.M.; Rech, G.E.; Rivera, L.P.; Benito, E.P.; Díaz-Mínguez, J.M.; Thon, M.R.; Sukno, S.A. Plant defense mechanisms are activated during biotrophic and necrotrophic development of Colletotrichum graminicola in maize. Plant Physiol. 2012, 158, 1342–1358.
- Thon, M.R.; Nuckles, E.M.; Takach, J.E.; Vaillancourt, L.J. CPR1: A gene encoding a putative signal peptidase that functions in pathogenicity of Colletotrichum graminicola to maize. Plant Microbe Interact. 2002, 15, 120–128.
- Fang, H.; Mullins, C.; Green, N. In addition to SEC11, a newly identified gene, SPC3, is essential for signal peptidase activity in the yeast endoplasmic reticulum. Biol. Chem. 1997, 272, 13152–13158.
- Meyer, H.A.; Hartmann, E. The yeast SPC22/23 homolog Spc3p is essential for signal peptidase activity. Biol. Chem. 1997, 272, 13159–13164.
- Eisermann, I.; Weihmann, F.; Krijger, J.J.; Kröling, C.; Hause, G.; Menzel, M.; Pienkny, S.; Kiesow, A.; Deising, H.B.; Wirsel, S.G.R. Two genes in a pathogenicity gene cluster encoding secreted proteins are required for appressorial penetration and infection of the maize anthracnose fungus Colletotrichum graminicola. Environ Microbiol. 2019, 21, 4773–4791.
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism—from biochemistry to genomics. Rev. Microbiol. 2005, 3, 937–947.
- Ludwig, N.; Löhrer, M.; Hempel, M.; Mathea, S.; Schliebner, I.; Menzel, M.; Kiesow, A.; Schaffrath, U.; Deising, H.B.; Horbach, R. Melanin is not required for turgor generation but enhances cell-wall rigidity in appressoria of the corn pathogen Colletotrichum graminicola. Mol Plant Microbe Interact. 2014, 27, 315–3
- Kou, Y.J.; Wang, S.P. Broad-spectrum and durability: Understanding of quantitative disease resistance. Opin. Plant Biol. 2010, 13, 1–5.
- Roux, F.; Voisin, D.; Badet, T.; Balague, C.; Barlet, X.; Huard-Chauveau, C.; Roby, D.; Raffaele, S. Resistance to phytopathogens e tutti quanti: Placing plant quantitative disease resistance on the map. Plant Pathol. 2014, 15, 427–432.
