Coronavirus disease 2019 (COVID-19) caused by the infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become the most severe health crisis, causing extraordinary economic disruption worldwide. SARS-CoV-2 is a single-stranded RNA-enveloped virus. The process of viral replication and particle packaging is finished in host cells. Viral proteins, including both structural and nonstructural proteins, play important roles in the viral life cycle, which also provides the targets of treatment. Therefore, a better understanding of the structural function of virus proteins is crucial to speed up the development of vaccines and therapeutic strategies. The structure-function correlation of viral proteins provides a fundamental rationale for vaccine development and targeted therapy.
- COVID-19
- SARS-CoV-2
- nonstructural proteins
- structural proteins
- vaccines
- therapy
1. Introduction of COVID-19
1.1. COVID-19 Pandemic
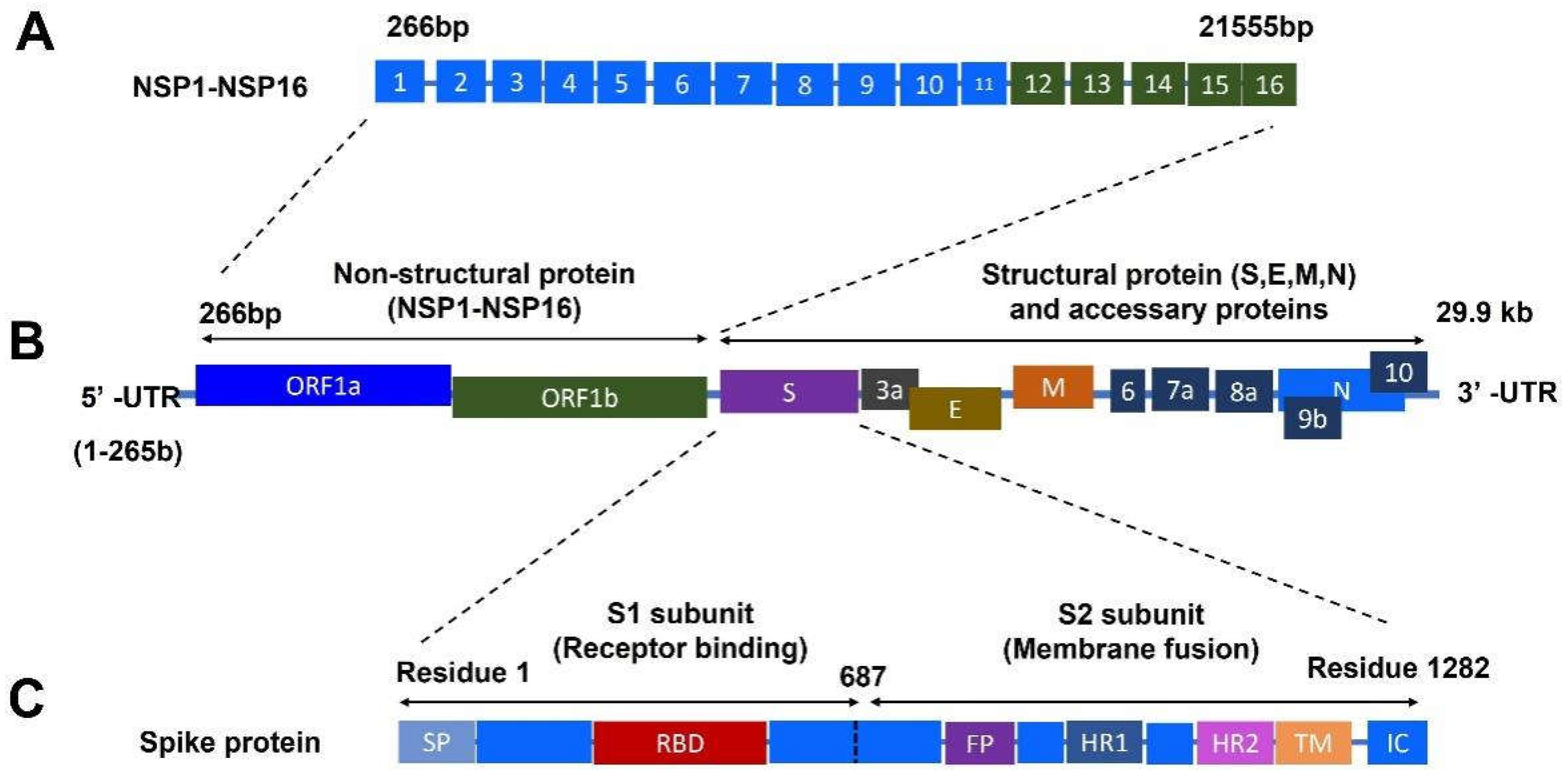
2. Vaccine Approach to Prevent Infection
A vaccine is one of the most effective preventative strategies to prevent highly contagious infections, including COVID-19 infection. Here, we discuss strategies to develop vaccines based on viral components.2.1. SARS-CoV-2 Inactivated Vaccines
2.1. SARS-CoV-2 Inactivated Vaccines
A viral vaccine is a vaccine that targets the whole virion, including the live-attenuated virus vaccine and inactive virus vaccine; both require the handling of viral infection agents for the purification of viruses to produce vaccines [8]. Live-attenuated vaccines have been successfully applied to protect against various diseases. However, the major disadvantage of their use is the potential reversion to gain virulence and become a disease-causing factor [9]. In contrast, an inactivated vaccine does not have the ability to revert to an unwanted state [10]. The whole inactivated SARS-CoV-2 virion that was generated in Vero cells has been used as an inactivated vaccine. The approved inactivated vaccines include the Sinopharm vaccine produced by the Beijing Institute of Biological Products Co., Ltd. (BIBP) (Beijing, China) [11][12], the Sinovac COVID-19 vaccine produced by Sinovac Life Sciences Co., Ltd. (Beijing, China) [13], and the COVAXIN vaccine developed by Bharat Biotech [14][15]. Vero cells have been applied to develop these vaccines. Other inactivated vaccines, such as the CovIran® vaccines investigated by Shifa Pharmed–Barkat, are waiting for clinical evaluation. The IMBCAMS vaccine is still under development [16]. There are many benefits of using inactivation technology for vaccine development as a traditional tool [17][18]. However, the development and production process of inactivated vaccines need more time due to the requirement for virus culture and inactivation procedure [19].2.2. Protein-Based Vaccines
Protein-based vaccines do not require handling of the live and attenuated viruses. These kinds of vaccines are stable and easy to distribute. NVX-CoV2373, developed by NOVAVAX, belongs to protein-based vaccines. The vaccine is composed of a nanoparticle core, around which is the recombinant protein antigen of SARS-CoV-2, the sequence of spike (S) protein from the strain Wuhan-Hu-1 (Figure 32A). The vaccine nanoparticles are then mixed with Matrix-M™ adjuvant to produce the ready-to-use vaccine products. An immunology study showed that this vaccine can provoke host protection by inducing robust immune responses, including both cellular and humoral immunity [20]. A clinical trial showed the protection efficacy was 90% against multiple mutations such as variants Alfa, Beta, and Delta. This vaccine has been approved for emergency use for the prevention of COVID-19 in many countries [21]. Other protein subunit-based vaccines either are under investigation or in ongoing clinical trials.
2.3. Nucleic Acid-Based Vaccines
2.3.1. DNA Vaccine
The nucleic-acid-based vaccines include DNA vaccines (e.g., DNA plasmids) and RNA vaccines. DNA vaccines do not contain any infectious pathogens. This is one of the safety advantages compared with weakened viral vaccines. DNA vaccines also have an advantage in stability compared with RNA vaccines [22]. Since the outbreak of COVID-19, different DNA-based vaccines have been explored and some of them put into clinical trials. According to the World Health Organization [16], most current DNA vaccines for SARS-CoV-2 are encoded for the S protein. However, there are differences in the vectors. For example, adenovirus ChAdOx1 is used as the vector for the AZD1222 Vaxzevria vaccine [23]. The adenovirus type 26 (Ad26) is utilized as a vector for the Janssen–Cilag International NV (Belgium) Ad26.COV2.S COVID-19 vaccine [24]. Human adenovirus has been applied as a vector for the development of the Sputnik V vaccine [25]. These aforementioned DNA vaccines have been approved for emergency use for the prevention of COVID-19. The adenovirus Type 5 Vector has also been used for the development of the Ad5-nCoV vaccine, which is under clinical trial evaluation [16].2.3.2. mRNA Vaccine
The mRNA vaccine has received considerable attention as a powerful tool to combat against the COVID-19 pandemic. Compared with the traditional vaccines, mRNA vaccines have irreplaceable advantages. For instance, mRNAs are faster and easier to produce. As soon as the virus sequence is decoded, the development of mRNA vaccines can be initiated in a time-saving fashion. There is no need to handle highly infectious pathogens, such as viral isolation and culture. However, some challenges for the development of mRNA vaccines also exist. The mRNA is not as stable as the DNA vaccine and is extremely sensitive to high temperatures, which places difficulty on vaccine distribution. The shortness of the half-life is the innate feature of mRNAs. Thus, the nucleotide needs to be modified to enhance its stability. The uridine modifications to reduce the unfavored immunogenicity to hosts also need to be considered for mRNA vaccine development [26]. Upon the outbreak of COVID-19, two mRNA vaccines were developed and broadly used for disease protection, including BNT162b2 from BioNTech/Pfizer [27] and mRNA-1273 from Moderna [28]. Both vaccines are nucleoside-modified mRNAs encapsulated by lipid nanoparticles as delivery vectors (Figure 32B). They are designed based on stabilized prefusion of the SARS-CoV-2 S protein (the full-length S protein). Clinical trials results show the protection efficacies of BNT162b2 (ClinicalTrials.gov, NCT04368728) [27] and mRNA-1273 (ClinicalTrials.gov, NCT04470427) [28] against SARS-CoV-2 infection were 95% and 94.1%, respectively.3. Antiviral Antibodies
Therapeutic antibodies for SAR-CoV-2 include a monoclonal antibody (mAb) and a cocktail of several monoclonal antibodies. Some antibodies are developed to block the binding site of the virus to the host. Others are developed for inflammatory modulation.3.1. Monoclonal Antibody
3.1.1. Monoclonal Antibody from Human Patients
Bebtelovimab (LY-CoV1404) is a mAb that neutralizes the SARS-CoV-2 spike glycoprotein RBD. It is a human immunoglobulin G-1 (IgG1) mAb. In vitro studies showed that it retained the binding to S proteins with a broad range of virus variants. Clinical evaluation on the effect of Bebtelovimab alone or together with other monoclonal antibodies Bamlanivimab and Etesevimab is ongoing [29][30]. It has been recommended as an alternative option for managing COVID-19 only when the preferred small molecules Ritonavir-boosted nirmatrelvir and Remdesivir are not available.3.1.2. Nanoparticle Antibody
Recently, Wu et al. characterized some RBD-specific neutralizing nanobodies and selected a nanobody library derived from alpaca immunization with the SARS-CoV-2 spike glycoprotein. The identified nanobodies showed a neutralizing function against the wild-type and Delta variants of SARS-CoV-2, which are specifically bound with a virus antigen [31]. Then, they also engineered a combined nanoparticle antibody using two nanobodies and a linker (Figure 32D), which showed a significantly increased neutralizing effect compared with every single particle [31]. These findings highlight the possibility of exploring the antibody from different sources and using multiple nanobodies.3.2. Antibody Cocktail
Due to the rapid mutation of viruses, the efficacy of mAbs is influenced. Thus, a cocktail of two or multiple antibodies may confer a powerful neutralizing effect against viral variants. Antibody cocktails for SARS-CoV-2 such as a cocktail of Tixagevimab plus Cilgavimab have been issued by FDA for emergency use against COVID-19 infection [32]. This cocktail also showed a neutralizing effect on Delta and Omicron variants [33][34]. There are many ongoing and finished studies for the efficacy evaluation of antibody cocktails. Some new approaches are worthy to specifically point out. One study reported a novel approach using protein structures to guide the design of antibody cocktails. The development of structure-guided antibody cocktails displays some great advantages. Some of these cocktails showed potent neutralizing ability against all the variants such as Alpha, Beta, Gamma, Epsilon, Iota, Kappa, and Delta in mouse and hamster models [35][36]. These cocktails hold promise and the potential to become one of the useful tools to combat viral infection, especially for the infection caused by highly mutated variants.3.3. Engineered Bispecific Monoclonal Antibody
A recent study reported a bispecific single-domain antibody, named bn03. It was designed based on the highly conserved region of RBD of Omicron variants. Interestingly, the bispecific bn03 showed a significantly higher naturalization efficacy compared with the cocktail combination of n3113v and n3130v. The EM-crystal structure analysis of Omicron (S-bn03) showed that it includes two arms, and both arms can simultaneously bind with the single RBD. The synergistic effect conferred by the two arms of bn03 increased the binding affinity of antibodies to viruses. Therefore, the neutralization effect of bn03 was increased. In addition, due to its small size, bn03 was able to bind to the most conserved cryptic epitopes in the deeper binding site. Moreover, the team verified that inhalation is the best method for bn03 delivery compared with other delivery paths [37]. Although there are some limitations such as limited sample numbers, this well-designed study still provides uresearchers positive inspiration for using a similar approach to engineer antibodies for virus treatment. In another study, two different bispecific antibodies were generated using a distinct design and were compared with their parental antibodies and their cocktails. One is tetravalent bispecific antibody 14-H-06, and another is a bivalent bispecific antibody 14-crs-06. Both in vitro and in vivo studies demonstrated that 14-H-06 coffered a significantly higher neutralization potency compared with 14-crs-06 (Figure 23E). Both bispecific (tetravalent and bivalent) antibodies showed greater efficacy compared with their parent antibodies or their cocktail [38]. Thise study illustrates that using an engineered bispecific antibody is a strategy to improve the efficacy of the parental antibody.
4. Peptide-Based Inhibitor
Small peptides can be designed and used as inhibitors to prevent the infection of viruses and other infectious pathogens [39][40]. For example, the N-terminal helix of the hACE2 contains the amino acids that mediated the interaction of hACE2 with the virus. This sequence has been used as a model to generate peptides that mimic the hACE2 (Figure 3F) [41]. The de novo design of miniprotein or peptides targeting the RBD binding site showed both increased binding affinity and enhanced stability. In the same study, the computationally generated scaffolds were also designed to stay around the ACE2 helix and interact with the virus RBD. In such a way, the scaffolds can block the direct interaction between ACE2 and virus RBD. The stability of the binding was further confirmed by the Cryo-EM structure [42]. Using the above-mentioned methods to design small peptides or miniproteins is another strategy to develop pharmaceutical medicines for viral infection (Figure 32F).References
- The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544.
- Dong, E.; Du, H.; Gardner, L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020, 20, 533–534.
- Ejaz, H.; Alsrhani, A.; Zafar, A.; Javed, H.; Junaid, K.; Abdalla, A.E.; Abosalif, K.O.A.; Ahmed, Z.; Younas, S. COVID-19 and comorbidities: Deleterious impact on infected patients. J. Infect. Public Health 2020, 13, 1833–1839.
- Machhi, J.; Herskovitz, J.; Senan, A.M.; Dutta, D.; Nath, B.; Oleynikov, M.D.; Blomberg, W.R.; Meigs, D.D.; Hasan, M.; Patel, M.; et al. The Natural History, Pathobiology, and Clinical Manifestations of SARS-CoV-2 Infections. J. Neuroimmune Pharmacol. 2020, 15, 359–386.
- Yadav, R.; Chaudhary, J.K.; Jain, N.; Chaudhary, P.K.; Khanra, S.; Dhamija, P.; Sharma, A.; Kumar, A.; Handu, S. Role of Structural and Non-Structural Proteins and Therapeutic Targets of SARS-CoV-2 for COVID-19. Cells 2021, 10, 821.
- Malik, Y.A. Properties of Coronavirus and SARS-CoV-2. Malays. J. Pathol. 2020, 42, 3–11.
- Wu, C.; Liu, Y.; Yang, Y.; Zhang, P.; Zhong, W.; Wang, Y.; Wang, Q.; Xu, Y.; Li, M.; Li, X.; et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B 2020, 10, 766–788.
- Murdin, A.D.; Barreto, L.; Plotkin, S. Inactivated poliovirus vaccine: Past and present experience. Vaccine 1996, 14, 735–746.
- Vetter, V.; Denizer, G.; Friedland, L.R.; Krishnan, J.; Shapiro, M. Understanding modern-day vaccines: What you need to know. Ann. Med. 2018, 50, 110–120.
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100.
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect. Dis. 2021, 21, 39–51.
- Hotez, P.J.; Bottazzi, M.E. Whole Inactivated Virus and Protein-Based COVID-19 Vaccines. Annu. Rev. Med. 2022, 73, 55–64.
- Li, X.N.; Huang, Y.; Wang, W.; Jing, Q.L.; Zhang, C.H.; Qin, P.Z.; Guan, W.J.; Gan, L.; Li, Y.L.; Liu, W.H.; et al. Effectiveness of inactivated SARS-CoV-2 vaccines against the Delta variant infection in Guangzhou: A test-negative case-control real-world study. Emerg. Microbes Infect. 2021, 10, 1751–1759.
- Sapkal, G.N.; Yadav, P.D.; Ella, R.; Deshpande, G.R.; Sahay, R.R.; Gupta, N.; Vadrevu, K.M.; Abraham, P.; Panda, S.; Bhargava, B. Inactivated COVID-19 vaccine BBV152/COVAXIN effectively neutralizes recently emerged B.1.1.7 variant of SARS-CoV-2. J. Travel Med. 2021, 28, taab051.
- Desai, D.; Khan, A.R.; Soneja, M.; Mittal, A.; Naik, S.; Kodan, P.; Mandal, A.; Maher, G.T.; Kumar, R.; Agarwal, A.; et al. Effectiveness of an inactivated virus-based SARS-CoV-2 vaccine, BBV152, in India: A test-negative, case-control study. Lancet Infect. Dis. 2022, 22, 349–356.
- WHO. Status of COVID-19 Vaccines within WHO EUL/PQ Evaluation Process; WHO: Geneva, Switzerland, 2021.
- Iglesias, E. Inactivated vaccines: A promising old tool against COVID-19. Res. Rev. Insights 2020, 4, 1–4.
- Iversen, P.L.; Bavari, S. Inactivated COVID-19 vaccines to make a global impact. Lancet Infect. Dis. 2021, 21, 746–748.
- Karch, C.P.; Burkhard, P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016, 120, 1–14.
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e811.
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F.; Chadwick, D.R.; Clark, R.; Cosgrove, C.; Galloway, J.; et al. Safety and Efficacy of NVX-CoV2373 COVID-19 Vaccine. N. Engl. J. Med. 2021, 385, 1172–1183.
- Chavda, V.P.; Hossain, M.K.; Beladiya, J.; Apostolopoulos, V. Nucleic Acid Vaccines for COVID-19: A Paradigm Shift in the Vaccine Development Arena. Biologics 2021, 1, 337–356.
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111.
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against COVID-19. N. Engl. J. Med. 2021, 384, 2187–2201.
- Jones, I.; Roy, P. Sputnik V COVID-19 vaccine candidate appears safe and effective. Lancet 2021, 397, 642–643.
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175.
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615.
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416.
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. bioRxiv 2022.
- Dougan, M.; Azizad, M.; Chen, P.; Feldman, B.; Frieman, M.; Igbinadolor, A.; Kumar, P.; Morris, J.; Potts, J.; Baracco, L.; et al. Bebtelovimab, alone or together with bamlanivimab and etesevimab, as a broadly neutralizing monoclonal antibody treatment for mild to moderate, ambulatory COVID-19. medRxiv 2022.
- Wu, X.; Cheng, L.; Fu, M.; Huang, B.; Zhu, L.; Xu, S.; Shi, H.; Zhang, D.; Yuan, H.; Nawaz, W.; et al. A potent bispecific nanobody protects hACE2 mice against SARS-CoV-2 infection via intranasal administration. Cell Rep. 2021, 37, 109869.
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Templeton, A.; Yuan, Y.; Seegobin, S.; Ellery, A.; Levinson, D.J. Intramuscular AZD7442 (Tixagevimab–Cilgavimab) for Prevention of COVID-19. N. Engl. J. Med. 2022, 386.
- Levin, M.J.; Ustianowski, A.; De Wit, S.; Launay, O.; Avila, M.; Seegobin, S.; Templeton, A.; Yuan, Y.; Ambery, P.; Arends, R.H. LB5. PROVENT: Phase 3 Study of Efficacy and Safety of AZD7442 (Tixagevimab/Cilgavimab) for Pre-exposure Prophylaxis of COVID-19 in Adults. Open Forum Infect. Dis. 2021, 8, S810.
- Fenwick, C.; Turelli, P.; Ni, D.; Perez, L.; Lau, K.; Lana, E.; Pellaton, C.; Raclot, C.; Esteves-Leuenberger, L.; Campos, J. SARS-CoV-2 Omicron potently neutralized by a novel antibody with unique Spike binding properties. bioRxiv 2022.
- Su, S.-C.; Yang, T.-J.; Yu, P.-Y.; Liang, K.-H.; Chen, W.-Y.; Yang, C.-W.; Lin, H.-T.; Wang, M.-J.; Lu, R.-M.; Tso, H.-C.; et al. Structure-guided antibody cocktail for prevention and treatment of COVID-19. PLoS Pathogens 2021, 17, e1009704.
- Liang, K.-H.; Chiang, P.-Y.; Ko, S.-H.; Chou, Y.-C.; Lu, R.-M.; Lin, H.-T.; Chen, W.-Y.; Lin, Y.-L.; Tao, M.-H.; Jan, J.-T.; et al. Antibody cocktail effective against variants of SARS-CoV-2. J. Biomed. Sci. 2021, 28, 80.
- Li, C.; Zhan, W.; Yang, Z.; Tu, C.; Hu, G.; Zhang, X.; Song, W.; Du, S.; Zhu, Y.; Huang, K.; et al. Broad neutralization of SARS-CoV-2 variants by an inhalable bispecific single-domain antibody. Cell 2022, 185, 1389–1401.e1318.
- Ku, Z.; Xie, X.; Lin, J.; Gao, P.; El Sahili, A.; Su, H.; Liu, Y.; Ye, X.; Li, X.; Fan, X.; et al. Engineering SARS-CoV-2 cocktail antibodies into a bispecific format improves neutralizing potency and breadth. bioRxiv 2022.
- Zhang, C.; Yang, M. Antimicrobial Peptides: From Design to Clinical Application. Antibiotics 2022, 11, 349.
- Basit, A.; Karim, A.M.; Asif, M.; Ali, T.; Lee, J.H.; Jeon, J.H.; Rehman, S.U.; Lee, S.H. Designing Short Peptides to Block the Interaction of SARS-CoV-2 and Human ACE2 for COVID-19 Therapeutics. Front. Pharmacol. 2021, 12, 731828.
- Karoyan, P.; Vieillard, V.; Gómez-Morales, L.; Odile, E.; Guihot, A.; Luyt, C.E.; Denis, A.; Grondin, P.; Lequin, O. Human ACE2 peptide-mimics block SARS-CoV-2 pulmonary cells infection. Commun. Biol. 2021, 4, 197.
- Cao, L.; Goreshnik, I.; Coventry, B.; Case, J.B.; Miller, L.; Kozodoy, L.; Chen, R.E.; Carter, L.; Walls, A.C.; Park, Y.J.; et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 2020, 370, 426–431.
