Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Brian Cameron Webb and Version 1 by Brian Cameron Webb.
There is a shortage of suitable tissue-engineered solutions for gingival recession, a soft tissue defect of the oral cavity. Autologous tissue grafts lead to an increase in morbidity due to complications at the donor site. Although material substitutes are available on the market, their development is early, and work to produce more functional material substitutes is underway. The latter materials along with newly conceived tissue-engineered substitutes must maintain volumetric form over time and have advantageous mechanical and biological characteristics facilitating the regeneration of functional gingival tissue.
- electrospinning
- gingival tissue
- material substitutes
1. Introduction
Gingival recession with tooth root exposure affects half of the adult U.S. population [1,2]. A more efficient and less painful solution to the current treatment standard could have a widespread impact, improving the lives of millions. Loss of gingival coverage around the tooth at the tooth–tissue margin is referred to as gingival recession and results in the exposure of the tooth’s root surface. This root exposure can lead to tooth sensitivity when eating, increased risk of biofilm accumulation and further tissue loss and aesthetic compromise. Tissue loss is primarily caused by inflammation associated with periodontitis (initiated from agents produced within plaque/biofilm) and mechanical trauma [3]. Not only does gingival recession yield challenges for the patients’ esthetic appearance, but it can also expose the roots surface of the tooth to cariogenic supragingival microbiota leading to an increased risk of dental caries and in the extreme case loss of tooth [3].
The current treatment for the soft tissue defect of gingival recession is primarily autologous soft tissue grafts, usually harvested from the patient’s palate [4]. However, material substitutes can be used in isolation, or with autologous grafts, and are available on the market, such as the Geistlich Fibro-Gide® bovine-collagen-based material [5]. This material still has limitations when compared to the gold standard of care (autologous grafts) [6], while several other more innovative materials that are now being studied and are discussed here. However, the field of tissue material substitutes [7], and tissue-engineered solutions is still in its infancy in this application area. The pain and length of recovery and the time to carry out the procedures could be greatly reduced, when compared to the standard-of-care-associated procedures, if superior scaffold material substitutes and/or pre-vascularized tissue-engineered constructs could be translated into the clinical realm [8,9]. Vascularized tissue-engineered substitutes hold the potential to provide the cells needed for tissue regeneration and anastomosis, and deliver novel scaffolding materials to promote their proliferation and phenotype expression towards successful tissue regeneration outcomes [10].
One promising processing method for fabricating materials for regenerating and/or engineering gingival tissue is electrospinning. The method enables the production of fiber and fibril features that are on the scale of those of host extracellular matrices (ECM). Despite its mention in a recent systematic review looking at engineering vascularized oral tissue (mainly gingiva and alveolar bone), the article provided no insight into the use of layered electrospun scaffolds, which is gaining interest by many tissue engineering groups attempting to replicate the ECM form and niche residence conditions for related cells to the tissue being grown [11]. It should be noted that while other examples of layered scaffolds for periodontal regeneration have been previously reported, none have addressed the potential use of electrospun elastomeric polymers [7].
2. Physiology and Disease of the Periodontium and Gingival Tissues: Defining Structure Requirements
The periodontium is comprised of four main tissue types: the alveolar bone, periodontal ligaments (connective tissue which allows for the attachment between the alveolar bone and root of the tooth), cementum, which is a mineralized tissue connecting the alveolar bone and the root of the tooth via periodontal ligaments, and gingival tissue which is the mucosal tissue that seals and protects the tooth from bacterial or physical threats as illustrated in Figure 1A [12]. The gingiva has two distinct layers, the epithelial tissue layer and the connective tissue layer (lamina propria) which make up approximately 30% and 70% of the gingiva, respectively [13,14]. The lamina propria can further be described as having two layers, the papillary layer, and the reticular layer [14]. The recession of gingival tissue is primarily caused by prolonged inflammation of periodontal tissue, periodontal treatment, and occlusal trauma [15]. Factors that could predispose an individual to gingival recession include a decrease in the thickness of the alveolar or buccal bone [15].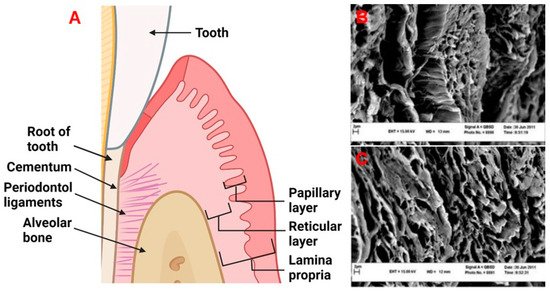
Figure 1. (A) The periodontal tissue anatomy. Created with BioRender.com. (B,C) Decellularized human gingival tissue adapted from previous literature reproduced under terms of the CC-BY license [16]. Copyright 2012, Nasser Mahdavishahri, Maryam Moghatam Matin, Masoud Fereidoni, Zahra Yarjanli, Seyed Ali Banihashem Rad, and Saeedeh Khajeh Ahmadi, published by Iranian Journal of Basic Medical Sciences. Created with BioRender.com, accessed on 8 April 2022.
Table 1. An outline of the vessels found within gingival tissue adapted from previous literature [24].
| Tissue Area | Type of Vessel | Diameter (µm) | Average Depth (µm) |
|---|---|---|---|
| Free gingiva | Capillary loops | ≤30 | 50–200 |
| Connective vessels | 50–100 | 200–700 | |
| Large blood vessels | 200–400 | ≥500 | |
| Attached gingiva | Capillary loops | ≤15 | 50–200 |
| Connective vessels | - | - | |
| Large blood vessels | 200–500 | ≥600 | |
| Alveolar mucosa | Capillary loops | ≤15 | 50–200 |
| Connective vessels | 200–600 | 200–700 | |
| Large blood vessels | ≥600 | ≥700 |
3. Current Material Options for Gingival Recession Treatment
The current treatment for gingival recession is typically autologous soft tissue grafts [4]. Additionally, material substitutes are available on the market, which have some reports on their efficacy. The two most common types of autologous grafts are connective tissue grafts (CTG) and free gingival grafts (FGG). CTGs involve harvesting connective tissue and grafting it such that root coverage and improved thickness of the gingival tissue are provided, as seen in Figure 2. An FGG entails harvesting connective tissue with surface epithelial tissue and placing it on the defect to cover the exposed root of the tooth and increase keratinized tissue [28]. Some of the major disadvantages of autologous gingival grafting are the increase in morbidity due to the harvest site, interindividual differences in terms of tissue availability, the time associated with the tissue harvesting (FGG takes ~25 min longer than using material substitutes) [29], donor infection, and bleeding from the harvest site [5,30].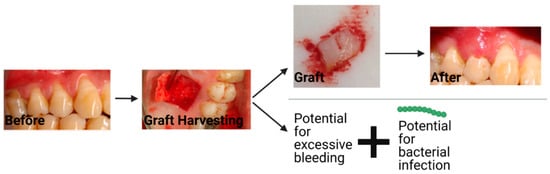
Figure 2. The workflow and potential complications that can occur with a connective tissue graft. The string of green dots represents bacteria. Created with BioRender.com with images from Dr. Michael Glogauer (University of Toronto) and images reproduced with permission under terms of the CC-BY license [31]. Copyright 2014, Sakshee Trivedi, Neeta Bhavsar, Kirti Dulani, and Rahul Trivedi published by the Journal of Clinical and Experimental Dentistry. Created with BioRender.com, accessed on 8 April 2022.
