Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Sandra Marcia Muxel and Version 2 by Catherine Yang.
In the past decade, microRNAs (miRNAs), a group of noncoding small RNAs, have emerged as functionally significant regulatory molecules with the significant capability of fine-tuning biological processes. The important role of miRNAs in inflammation and immune responses is highlighted by studies in which the regulation of miRNAs in the host was shown to be related to infectious diseases and associated with the eradication or susceptibility of the infection.
- microRNAs
- gene expression
- post-transcriptional
1. Introduction
The discovery of noncoding RNAs (ncRNAs) has revolutionized the field of molecular biology. These ncRNAs do not code for proteins but globally impact genome maintenance and gene expression [1][2][3][4][1,2,3,4]. These RNAs can be categorized according to their length, localization, and function, such as ncRNAs that regulate gene expression, as long noncoding RNAs (lncRNAs), microRNAs (miRNAs), small interfering RNAs (siRNAs), and PIWI-interacting RNAs (piRNAs); RNA maturation, as small nucleolar RNAs (snoRNAs) and small nuclear RNAs (snRNAs); and protein synthesis, as ribosomal RNA (rRNAs) and transfer RNAs (tRNAs) [5][6][5,6].
miRNAs (typically 21 nucleotides) are a class of ncRNAs which interact with the 3′ untranslated region (3′ UTR) of messenger RNAs (mRNAs), leading to mRNA degradation or translational repression [7][8][9][10][7,8,9,10]. The involvement of miRNAs in a variety of biological processes, such as chronic pathologies and infectious diseases, illustrates their complexity as they have been described as being able to simultaneously interact with diverse molecules, such as RNA binding proteins (RBPs), making the miRNA recognition site more accessible to the RNA-induced silencing complex (RISC), which also enables the processing of pri-miRNAs [11][12][11,12] or lncRNAs [1][13][1,13], controlling different points of the gene expression flux.
Host–pathogen interactions result in signaling and physiological modifications in host cells that induce the miRNA-mediated post-transcriptional regulation of genes involved in the inflammatory response during the induction of the immune response [14][15][14,15]. These miRNAs are involved in the modulation of both innate and adaptive immune responses [16]. In recent years, the alteration of miRNA expression has been studied extensively in cancer or infectious diseases caused by bacteria, viruses, and parasites [17][18][19][20][17,18,19,20].
The proposition of miRNAs as potentially non-invasive biomarkers for clinical diagnosis of diseases, using patient plasma, has prompted descriptions of tumor-derived miRNAs in that medium [21][22][21,22]. Also, some studies have shown that miRNAs are differentially expressed in patients with hepatotoxicity [23], artery disease [24], and infectious diseases [19][20][25][19,20,25].
2. miRNAs—Biogenesis and Gene Expression Regulation
miRNAs are transcribed from intergenic regions as either monocistronic or polycistronic (miRNA-clusters) transcripts [26][27][28][26,27,28]. Those sequences can have their own promoter region or can depend on the transcription of host genes if they are intragenic [26][27][28][26,27,28]. They can be encoded in exonic or intronic regions and transcribed in the same direction as that of the pre-messenger RNA, leading to the use of the promoter region of mRNAs for their transcription [29] (Figure 1). miRNAs are transcribed by RNA polymerase II and fold into long double-strand primary miRNA transcripts (pri-miRNA) [7]. In the nucleus, the class 2 RNase III DROSHA and DGCR8 (a double-strand RNA-binding protein, also known as Pasha) complex recognizes features in the hairpin structures of pri-miRNA and processes the molecule to form the precursor miRNA transcript (pre-miRNA) [30][31][30,31]. The pre-miRNA is coupled with the Exportin 5 protein and exported to the cytoplasm [32] where an RNase III family protein Dicer complexed with TRBP (transactivation response element RNA-binding protein) recognizes and processes the pre-miRNA into the miRNA-duplex, a mature miRNA [33][34][33,34]. The functional strand of the mature miRNA is loaded into the RISC, coupled with the argonaute (AGO) protein family as important components of ribonucleoprotein (RNP) complexes (miRNPs) which guide interactions with the target mRNA, leading to the regulation of gene expression [33][35][36][37][33,35,36,37].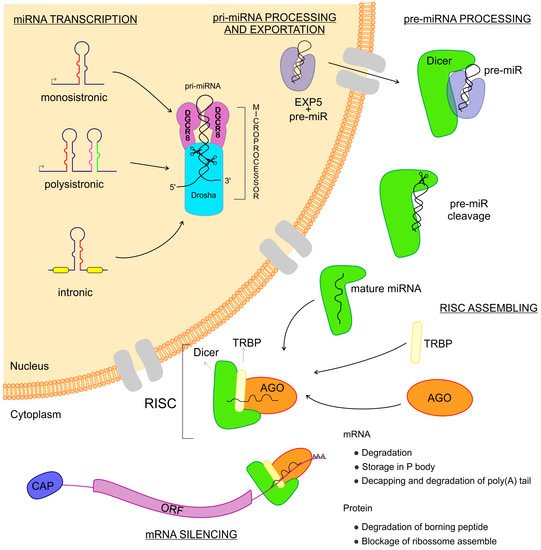
Figure 1. Biogenesis of microRNAs (miRNAs). The miRNAs are small non-coding RNAs transcribed from DNA sequences which can be monocistronic or polycistronic, comprised within exons, introns, or a unique host gene. Right after the transcription, this new RNA sequence is called primary-miRNA (pri-miRNA), which is folded in a hairpin conformation and coupled with the microprocessor: a combination of DGCR8 and Drosha RNases which cut the pri-miRNA, making it a pre-miRNA. Afterward, the pre-miRNA is coupled to Exportin 5 and then exported to the cytoplasm where this complex is found by Dicer, which cuts the pre-miRNA, releasing two mature miRNA arms. The mature miRNA complexed to the Dicer can now couple with the Argonaute and TRBP proteins, thus assembling the RNA Induced Silencing Complex–RISC. When the RISC is done, it can find the messenger RNAs that are the targets to the miRNA complexed. Once found, the message is now silenced and the gene expression regulated.
miRNAs regulate gene expression at a post-transcriptional level in a sequence-specific manner, exerting a massive biological impact [7]. Research has been dedicated toward attempting to understand the mechanisms of post-transcriptional regulation mediated by miRNAs, with studies performed in vitro, in vivo, and in cell-free extracts, as well as using bioinformatics prediction tools to demonstrate the putative regulation, by miRNAs, of nearly 30% of all protein-coding genes in mammalian cells [38]. miRNAs can regulate translation by (a) repressing the initiation of protein translation of 7-methylguanosine (m7GpppN)-capped mRNAs, inhibiting the binding of the eukaryotic translation initiation factor (eIF) subunits eIF4E, eIF4F, and eIF4G, which promote the scaffolding of mRNA for association of the ribosome initiation complex [39][40][41][39,40,41]; (b) preventing the association of ribosome 60 S subunit with 40 S and mRNA via interference of the binding between eIF6 and 60 S, which can be mediated by the AGO2–Dicer–TRBP complex [42]; and (c) blocking elongation via the miRNA–mRNA association with active polysomes, impacting the post-initiation step of translation [43]. miRNAs can also regulate mRNA destabilization by recruiting decay machinery components, leading to mRNA poly(A) tail deadenylation by exonuclease activity in 3′→5′ degradation, or decapping followed by 5′→3′ exonuclease activity [44][45][44,45]. In addition, miRNAs are secreted into vesicles, as exosomes, or into extracellular fluids and circulation, highlighting the potential roles of extracellular miRNAs in mediating intercellular communications and as biomarkers for a variety of diseases, including infectious diseases [46].
