The COVID-19 pandemic has affected many people in general and athletes in particular. This has led to a series of restrictions, which from a pathophysiological point of view, may affect the athlete’s performance in the short and long term. The restrictions basically affect training and eating habits, disturbing physical condition, as well as psychological behavior and general health status. Several aspects of systemic alterations caused by the SARS-CoV-2 virus and the resultant COVID-19 disease have been currently explored in the general population. ResearchersHowever, very little is known about these particular aspects in sportsmen and sportswomen. We believe that the most important element to take into account is the neuromuscular aspect, due to the implications that this system entails in motion execution and coordination. In this context, deficient neuromuscular control when performing dynamic actions can be an important risk factor for injury.
1. Introduction
The COVID-19 pandemic has affected many people in general and athletes in particular. This has led to a series of restrictions, which from a pathophysiological point of view, may affect the athlete’s performance in the short and long term. The restrictions basically affect training and eating habits, disturbing physical condition, as well as psychological behavior and general health status
[1][2][3][4][5][1,2,3,4,5].
The disease caused by SARS-CoV-2 infection, known as COVID-19, is accompanied by mild symptoms with fever, cough, myalgia, fatigue, mild dyspnoea, sore throat, and headache. Nevertheless, great variability of symptoms from one person to another has been reported. Since COVID-19 is an emerging infectious disease, an additional problem is that the long-term effects and sequelae of the disease are unknown, both for moderate and severe forms
[6]. Moreover, the psychological component must be considered, because it can condition many biological responses. In this regard, i.e., in the context of disasters and traumatic events, significant increases in stress levels have been observed in the population
[7][8][7,8]. The same results were obtained from the first countries affected by the SARS-CoV-2 virus, such as China and South Korea
[9][10][9,10]. The state of stress resulting from the pandemic and restrictive measures has long-term health effects, with an increased risk of physical and mental illness affecting sportsmen and sportswomen as well
[11][12][13][14][15][11,12,13,14,15]. Even in the absence of pandemic situations, chronic stress is a major public health concern
[16]. Moreover, it should be kept in mind that SARS-CoV-2 infection not only affects the pulmonary and heart systems, but also other organs such as the liver and kidneys
[17][18][19][17,18,19]. All these disturbances must have an impact on sports performance, particularly among professional sportsmen and sportswomen
[20]. In this line, physical performance is a complex concept that takes into account many aspects, including
[21]: (a) efficient energy production (aerobic and anaerobic); (b) neuromuscular dynamics (strength and technical skills); and (c) psychological features (motivation and strategies). The COVID-19 pandemic has disturbed this framework.
2. Respiratory Disturbances
It has been well established that reduction of physical activity impairs correct glycemic control and changes body composition, favoring the increase in fat mass (FM) and reducing muscle mass (MM), all with negative consequences on maximal oxygen uptake (VO
2max)
[22][23][31,32]. This situation could be aggravated in athletes that have undergone SARS-CoV-2 infection
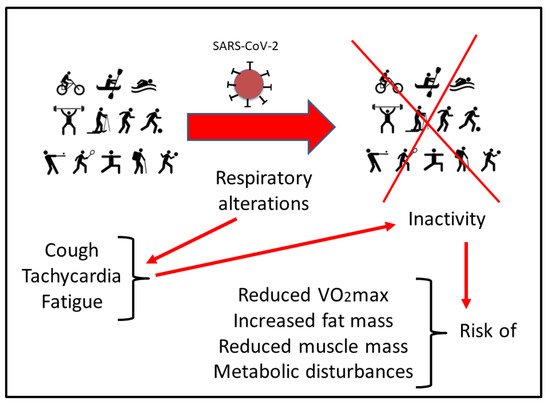
[24][33]. In addition to the metabolic changes and psychological impact due to inactivity, respiratory alterations have to be considered. Many athletes reported residual symptoms even months after the initial COVID infection, including a persistent cough, tachycardia, and fatigue. This situation makes it difficult to return to physical activity due to the sustained demand of the respiratory system for optimal sports performance, particularly in aerobic disciplines
[25][23]. In addition, maintaining a relatively stable but at the same time adapted metabolism during exercise poses a major challenge to respiratory and circulatory functions
[26][34]. Therefore, programmed physical activity is instrumental during post-infection recovery in order to improve oxygen uptake, healthy circulating metabolic parameters, energy balance, and metabolic control. However, safe recommendations under physician supervision and in base on the new evidence have to be updated for athletes who have suffered the disease when returning to competition. Altogether, COVID-19 has an impact on metabolic adaptation during exercise
[27][35]. Therefore, athletes with COVID-19 disease display an increased risk of reduced maximal and submaximal performance as well as altered cardiovascular and muscle metabolic adaptations
[28][36] (
Figure 1).
Figure 1. Scheme of the possible risks that can derive from the residual symptoms in respiratory function after SARS-CoV-2 infection. See the text for more details.
3. Muscular Repercussions
SARS-CoV-2 is capable of infecting multiple cell types with a preference for pulmonary epithelium and immune cells
[29][37]. As a result, muscle pain (myalgia) and fatigue are usual initial symptoms of the disease, occurring in 35% of COVID-19 patients
[30][31][38,39]. In general, these findings have been associated with critically ill myopathy and steroid myopathy associated with the clinical picture of COVID-19. This muscle deterioration could be explained through the activation of angiotensin-converting enzyme 2 (ACE2), a membrane-attached protein that mediates the entry of SARS-CoV-2. In this context, studies performed in animal models show that activation of ACE2 induces skeletal muscle alterations and reduces exercise capacity, with mitochondrial dysfunction and decreased oxidative fiber number, resulting in subsequent muscle atrophy
[32][40].
Several factors may play a key role in muscle plasticity. One of them seems to be the degree of mechanical loading
[33][41]. In this context, inactivity decreases the mass and size of muscle fibers and consequently leads to weakening
[34][42]. Thus, it can be hypothesized that the acute inflammatory response to COVID-19 infection would consume the proteins that work as building blocks for muscle activity. As documented in other inflammatory processes, the synthesis of acute-phase proteins, such as C-reactive protein (CRP), ferritin, tumor necrosis factor-α (TNF-α), and the different interleukins (ILs), could appear concomitantly with albumin and muscle protein degradation. Nevertheless, this hypothesis needs to be investigated in future research.
Myalgia and fatigue are frequently observed in 44–70% of patients with COVID-19
[35][43], being the fifth most common symptom in patients with COVID-19
[30][38]. Several studies in patients infected with other coronaviruses have shown that myalgia increased serum CK concentrations
[36][44] or produce rhabdomyolysis
[37][45] in 1/3 of infected individuals. However, in the case of COVID-19, the circulating CK levels were close to baseline values (around 200 U/L), making it impossible to differentiate between a myogenic or neurogenic alteration
[38][39][46,47]. It can be hypothesized that the “cytokine storm” induced by SARS-CoV-2 could be a possible mechanism underlying the persistent myalgia and fatigue
[40][48], but this statement needs further research.
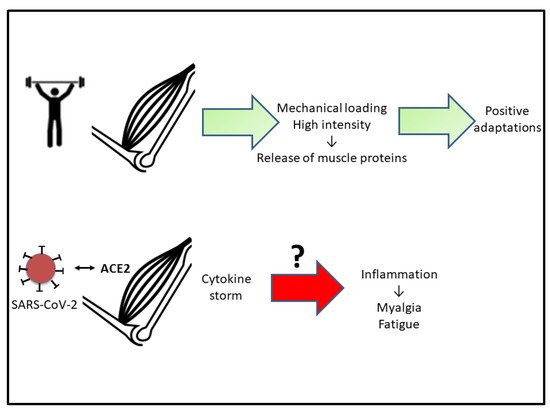
Under normal physiological conditions, intense and sustained exercise causes muscle damage with the release of muscle proteins leading to inflammation of myocytes and altered muscle integrity. This inflammatory response leads to increases in circulating muscle proteins, such as CK, lactate dehydrogenase (LDH), and myoglobin (Mb) as well as in pro-inflammatory cytokines, including TNF-α and IL-6
[41][42][43][49,50,51]. In the context of COVID-19, a similar inflammatory reaction occurs, together with high levels of acute phase reactants such as ferritin and CRP
[44][52]. Increased pro-inflammatory cytokines are involved in the induction and effector phases of all immune and inflammatory responses
[45][53]. In addition, the stress component that occurs in this situation enhances the synthesis of glucocorticoids. In this context, it exists a relationship between the immune system function, the inflammatory response, and the hypothalamic-pituitary-adrenal (HPA) system
[46][47][48][54,55,56].
The muscle inflammatory process is associated with increased wasting, loss of strength, and functional damage
[49][57]. One of the consequences is increased muscle fatigability. Fatigue is accompanied by the release of proteins and enzymes into the bloodstream: CK, Mb, and LDH. These proteins are indicators of muscle damage and muscle stress associated with intense exercise
[50][58] (
Figure 2). In the field of sport, it is known that exercise increases the activity of enzymes involved in glycolysis and glycogenolysis such as glycogen phosphorylase, phosphofructokinase, and LDH
[51][52][59,60]. Therefore, an interesting hypothesis to check would be to investigate if the cumulative tiredness and metabolic demand that occurs during COVID-19 could be similar to the fatigue situation undergone after very intense exercise execution. Izquierdo et al.
[53][61] observed that after a period of short-term strength training, exercise-induced loss of functional capacity occurred in athletes.
Figure 2. Scheme indicating that adequate physical activity results in positive muscle adaptations. The hypothesis to test (?) is if the inflammation associated with COVID-19 is responsible for myalgia and muscle fatigue. See the text for more details. Abbreviations used: ACE2, angiotensin-converting enzyme 2.
4. Cardiac Consequences
It has been established that COVID-19 leads to cardiac and vascular complications. A possible link to the above-mentioned “cytokine storm” is hypothesized
[54][65]. “Cytokine storm” occurs in the severe phase of COVID-19 and could lead to impaired cardiac function, presenting characteristics similar to those reported in classic forms of stress or catecholamine-induced cardiomyopathy
[55][66]. As indicated, this is more noticeable in severe COVID-19 cases, particularly in subjects with comorbidities, such as hypertension, type 2 diabetes, and cardiovascular disorders
[56][67]. In addition, it seems that SARS-CoV-2 may directly infect cardiomyocytes, causing myocarditis with acute and severe deterioration of cardiac function
[56][67].
From the data reported so far by different media, many professional team sports players have been infected with SARS-CoV-2. According to some reports, athletes appear to be at a higher risk of developing myocarditis than the general population, although there is no evidence to support these claims. It is true that intense and sustained exercise may influence susceptibility to infection, depending on the intensity and duration of physical activity
[41][42][57][58][49,50,68,69].
Myocarditis associated with COVID-19 has been reported in almost 1/5 of patients, with a 50% of survival rate
[59][70]. Myocarditis has traditionally been considered the main cause of life-threatening ventricular arrhythmias in sportsmen
[60][61][62][71,72,73]. Therefore, this incidence would support the hypothesis of the development of cardiomyopathies in athletes suffering from COVID-19. It is well established that after a long period of inactivity, aerobic capacity (according to VO
2max) can decrease significantly, accompanied by an increase in heart rate
[27][63][35,74]. These physiological changes lead to a decrease in muscle capillaries and a loss of sensitivity in the mechanisms that control body temperature
[64][65][75,76]. As mentioned before, different outcomes have been reported by diverse aerobic training protocols during COVID-19 lockdown
[5][66][67][5,63,64].
Taking into account the novelty and limited knowledge of COVID-19, the cardiomyopathy prevalence and clinical implications (acute and late) are largely unknown. Therefore, the incidence of myocardial affectation, which in many cases may be silent for a long period of time after the resolution of typical COVID-19 symptomatology, is also unknown.