In line with the recent industrial trends of hyperconnectivity, 5G technology deployment, the Internet of Things (IoT) and Industry 4.0, the ultimate goal of corrosion prevention is the invention of smart coatings that are able to assess their own condition, predict the onset of corrosion and alert users just before it happens. It is of particular interest to tackle corrosion that occurs in non-accessible areas where human inspectors or handheld devices are useless. To accomplish this, a variety of technologies that are embedded or could potentially be embedded into the coatings are being developed to monitor coating condition, which are based, for instance, on the evolution of electrochemical or mechanical properties over time. For these technologies to be fully embedded into the coatings and work remotely, solutions are needed for connectivity and power supply. A paradigm shift from routine prescheduled maintenance to condition-based preventive maintenance could then become a reality.
- Organic Coating
- Health Monitoring
- Evolution
1. Evolution of the Electrochemical Properties of Coatings
1.1. Electrochemical Impedance Spectroscopy (EIS)
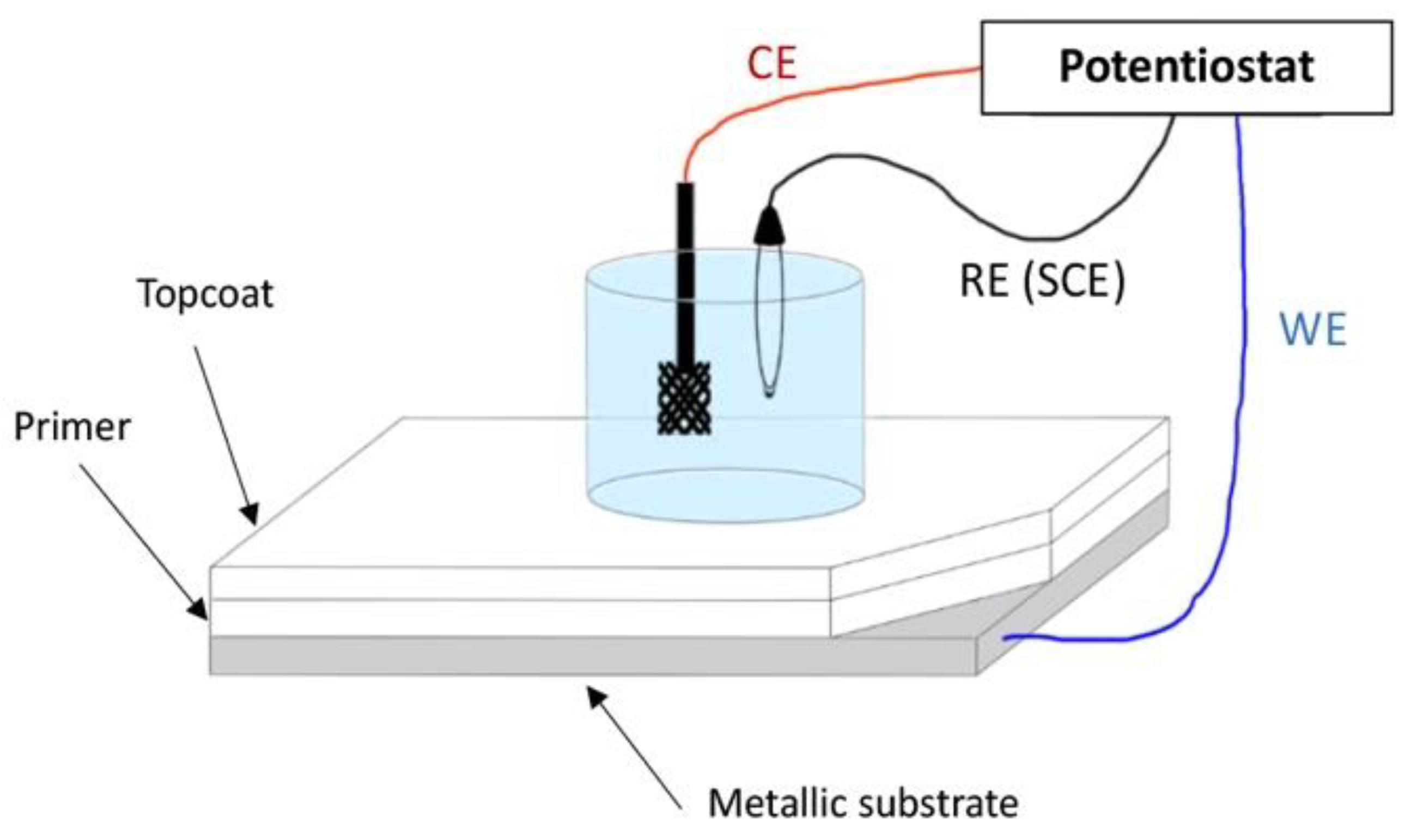
-
The uptake of moisture by the coating,
-
Corrosion incubation time,
-
The corrosion of the substrate.
1.2. Electrochemical Noise Measurements (ENM)
ENM monitors the small potential and current fluctuations that occur naturally in electrochemical cells to evaluate corrosion processes and coating states, with the latter being the focus of this work. Several advantages and disadvantages are often noted when ENM is compared to EIS. First of all, ENM does not require a sinusoidal perturbation; therefore, it is considered less intrusive than EIS [73][20]. In the same work, Bierwagen et al. stated: “the primary reason for the failure of EIS methods in cyclic exposure conditions…” (similar to those encountered by coatings in-service) “…is that in the potentiostatic mode, all measurements are made about Ecorr of the system under investigation, and if Ecorr is time dependent at a rate that exceeds the lowest frequency of the EIS measurements, the system is non-stationary and applying EIS techniques give errors” [73][20]. Therefore, when the corrosion potential of a system is not stable, the measurements of EIS at low frequency are often erroneous. Thus, ENM measurements have proved to be more accurate and quicker for gathering data than EIS. Nevertheless, ENM has drawbacks that can lead to a greater variance [84][30] and the results are based on a more complex theoretical foundation than those of EIS [73][20]. Iverson [85][31] first found a correlation between electrode potential fluctuations and corrosion processes, whereas Eden and Skerry [86][32] first applied the technique to coated metals. The most common representation of the fluctuations, also named noise, in voltage (V) and current (I) that are recorded in ENM is noise resistance, Rn. It is calculated as the ratio of the standard deviation of the voltage noise to the standard deviation of the current noise [87,88][33][34]: Rn = σV (t)/σI (t). Experimentally, Rn is equivalent to the low-frequency impedance, |Z|0.01Hz, that is obtained from EIS [89][35]. As in EIS, a decrease in Rn is indicative of increased coating degradation due to the advancement of water, ions, and other destructive species into the coating system. Other interesting papers on ENM theory fundamentals, application and results interpretation can be found in the following references: [69,90,91][16][36][37]. A salt bridge enables the current to flow between the two samples. The CE lead is connected to one substrate and the WE lead is connected to the other. A laboratory RE is needed in one of the electrolyte cells. The use of a zero-resistance ammeter (ZRA) allows the user to keep the potential difference at zero during measurement. The early ENM montage was satisfactory for laboratory use but was clearly not suitable for in-service monitoring or quality control. Mabbutt and Mills [92][38] soon realised the need for montage simplification and introduced an alternative experimental setup, which eliminated the need for two isolated specimens and the salt bridge. Their double reference electrode setup reduces preparation time and set the basis for embedded electrode techniques. This device is composed of a single substrate/sample with two electrolyte immersion cells and a support that is connected to the RE lead, whereas the reference electrodes replace the previous WE lead. Indeed, the Mabbutt and Mills setup is often referred to as a “reverse configuration”, as all of the electrical components are reversed. Subsequently, reverse ENM experiments [90,93][36][39] can proceed without a connection to the substrate, in which three lab electrodes are electrically isolated by the electrolyte immersion cells. This set the basis for the in-situ application of ENM, in which a connection to the substrate is not feasible (no connection to the substrate, NOCS). In [94][40], Mills et al. used single substrates in operation with an electrolyte-soaked filter paper instead of the electrolyte immersion cell. A copper foil that was used as pseudo reference electrode was placed on the filter paper and taped to hold it steady. Suitable results from the ENM measurements were obtained by connecting this to the electrode. ENM measurement using the reverse configuration was proceeded by replacing the lab reference electrodes with an embedded Pt mesh. ENM was also successfully operated by Wang et al. [73][20], who placed Pt wire electrodes inside the coating to characterise the organic coatings. This in situ configuration can be used for continuous measurement if the humidity does not compromise the conductivity requirements. Su et al. [95][41] studied the AC–DC–AC-accelerated weathering of aircraft and industrial coatings using ENM with embedded Pt foil leaf electrodes. Subsequently, the materials were also aged by thermal cycling [96][42] and prohesion [69][16]. They also compared the EIS measurements to reverse configuration ENM results. In [69][16], a novel electrochemical noise (EN) setup was used with embedded electrodes (EEs), which was found to be suitable for the in situ testing of the integrity of organic coatings when submitted to a marine alternating hydrostatic pressure (AHP) environment. The analysis of the EN results from the EE configuration were compared to those obtained from a conventional EIS configuration. Moreover, the corrosion behaviour of the substrate below the coating was analysed to determine the performance of the protective coating. The results confirmed that the in-situ EE configuration under AHP is a valid and reliable approach. Another versatile and quick technique that can be used to determine whether there are defects in coatings and the level of protection that is available is electrochemical noise measurement [69,88][16][34]. However, it has been noticed that with this device, the data analysis is more complicated in passive and inhibited systems and that the collection of data and the choice of methods for analysis are determinant in the effectiveness of the technique [84,90][30][36]. Additionally, as for EIS, ENM is sensitive to external electromagnetic fields and needs quite complex instrumentation to overcome these perturbations.1.3. Potentiodynamic Polarisation Measurement (PDP)
This kind of measurement belongs to one of the most commonly used DC electrochemical methods for corrosion measurement. The polarisation curve can be used to determine the corrosion potential and the corrosion rate of the metal under the given conditions (Tafel slope). The advantage of this method is reflected in the possibility of localised corrosion detection, the easy and quick determination of the corrosion rate and the efficiency of the corrosion protection. More details can be found in the book chapters of Vastag et al. [97][43] and Atta Ogwu et al. [98][44].2. Evolution of the Internal Stress–Strain State of Coatings
“A direct measurement of internal stresses would be necessary to go deeper in the understanding of the coating degradation modes” [37][45]. In their article, Perrin et al. studied the influence of the alternation frequency of different weathering conditions on the mechanisms and rate of coating degradation. The studied coating was a three-layer system composed of an epoxydic primer and basecoat and an alkyd top-coat on steel substrates. It was found that the frequency of change between different conditions, such as immersion/emersion or hot/cold, had a greater impact on the coating degradation than the duration of each different step. In other words, degradation was greater and faster when samples underwent different conditions successively compared to when they were kept in a single type of environment, even when they were kept there for a longer cumulative period. It was hypothesised that this could be related to the impact of alternating between different environments on the mechanical properties of the coating’s polymeric binder. Indeed, right after the coating is applied and cured, the polymeric network is at its maximum internal stress state due to shrinkage being prevented by surface interlocking and bonding forces. With exposure to the environment and time, the internal stresses of the polymeric network progressively relax. In the long term, surface cracks can develop because of such relaxations. This, in turn, further facilitates the ingress of water molecules into the coating, which induces swelling in the polymeric network and changes the coating’s internal stress–strain state because of plasticisation. When corrosion subsequently develops at the coating–substrate interface and corrosion products accumulate underneath the coating, strains result in the vicinity of the cracks. One common trend can be observed in all these situations: each stage of the coating’s lifetime has an impact on the physical and mechanical condition of the coating and its polymeric network. Therefore, it seems reasonable to assume that when the coating’s internal mechanical state and its evolution over time could be monitored, it may be possible to correlate internal stress–strain changes with the events that were responsible for that change; thus, it may be possible to follow the coating’s condition in real time. From these findings, a question can be raised: is it possible to measure changes in the internal polymer stress–strain state of a coating accurately enough to detect the changes that were induced by each of those events? Commonly, strain is measured by gluing metallic strain gauges onto a substrate. This, however, has inherent disadvantages in terms of forced coupling between the surface strain of the bent substrate and the glued strain gauge sensors [91][37]. The fact that, in general, the glue and the strain gauge substrate have different moduli of elasticity affects the maximum achievable sensitivity [91][37]. Moreover, since the sensors are attached to the surface, their monitoring range is practically limited to the coating’s surface. However, several different technologies that approach the problem with various original technical means have been proposed by the scientific community and are presented below.2.1. Optical Fibres–Fibre Bragg Gratings
A fibre Bragg grating (FBG) is a short portion of optical fibre, in which a certain pattern that induces periodic changes in the refractive index of light has been created. This acts as an optical filter that reflects some wavelengths and transmits others [99][46]. Reflected and transmitted wavelengths depend on the spacing of the patterning. When such an optical fibre is embedded into a coating, typical coating-related phenomena, such as swelling due to water absorption, deformation due to osmotic blistering or delamination, can alter the distance of the Bragg grating patterning, which results in changes to the reflected/transmitted wavelengths. In other words, changes in the coating strain result in a shift in the Bragg grating wavelengths. Ramezani-Dana et al. [101][47] presented a technique based on fibre Bragg grating (FBG) that is capable of accurately measuring mechanical strains inside polymeric composite materials. The technology is used to monitor the ingress of water into the laminate composites by tracking the water-induced swelling of such materials and to estimate their moisture expansion coefficients. The FBG sensors are embedded in between the composite layers. By inscribing several FBGs with different grating periods within the same optical fibre, an array of gratings was manufactured, which allows different positions within the structure to be monitored with a single sensor line. Similarly, Marro Bellot et al. [29][48] used low-cost optical fibre sensors (OFS) that were embedded in epoxy matrices to monitor water diffusion into the matrices. To fabricate the single-ended evanescent wave OFS that was used in that study, standard glass optical fibres were chemically etched to expose the core, within which the light was confined, to the surrounding environment. Initially, 125 µm diameter optical fibre wires were reduced to a 50 µm diameter by etching their coating. The etched wires were then embedded into glass fibre-reinforced epoxy matrices and the samples were immersed in simulated sea water at 80 °C. The sensors were interrogated using a low-cost benchtop spectrometer that worked in the near-infrared spectrum. Optical fibre-based technologies for SHM are experiencing a strong expansion due to their advantages over other kinds of technologies. As they are based on optical properties, they are not susceptible to electromagnetic fields, they show a high sensitivity of measurement and they do not conduct electricity because they are made of inorganic non-metallic materials, all of which only allows the propagation of light along the fibre. OFSs have been successfully tested in extremely hazardous environments, such as high and low temperatures and pressures, very corrosive media, radioactive zones, etc. With a single OFS, it is possible to perform measurements at different distant points (remote sensing).2.2. Embedded Piezoresistive-Based Strain Gauges
In their work, Enser et al. [102,103,104,105][49][50][51][52] seemed to adapt a promising existing technology [106,107][53][54] that has recently been applied to structural health monitoring (SHM) for use in organic coatings. Enser et al. used the piezoresistive properties of certain types of nanocomposites to fabricate an internal strain gauge by printing the sensing part inside a coating, sandwiched in between the basecoat and top-coat layers. By doing so, the main drawbacks that are associated with surface-attached gauges can be circumvented. There is no longer a need for a glue and there is a very reduced geometrical distance to the substrate, which thus improves the force coupling between the substrate and the sensor and achieves a higher gauge factor. Furthermore, the strain gauges are easily and inexpensively made by screen-printing electrically conductive ink-based sensing electrodes onto precoated steel substrates prior to the addition of a stabilising top layer, which closes the coating “sandwich”. The reseauthorchers compared two different types of inks: silver-based and carbon-based. The silver-based ink showed a gauge factor that was similar to that of a common surface-glued gauge and a good linearity with temperature, while the carbon-based ink showed a gauge factor that was almost three times higher than that of a common gauge but its temperature coefficient was only approximately linear with temperature in a very small temperature range [105][52]. In any case, a temperature shift correction has to be applied to this kind of strain sensor, as temperature affects the piezoresistive operating principle. Enser et al. used their mechanical sensors to study the bending of coated steel cantilevers and to fabricate integrated capacitive touch sensors as well [108][55]. However, can this kind of coating-integrated strain sensor be used to monitor the physical properties of a coating in the long term? Instead of using them to detect induced bending deformations at a given punctual time, can this piezoresistive-based technology track the internal stress–strain evolution that occurs through the lifetime of a coating as it ages? In other words, can they be used to sense, for example, coating swelling that was caused by water absorption or strains that were induced by the formation of blisters in the coating? If this were possible, advantages over the previously reviewed electrochemical-based techniques or optical fibres could be numerous, for instance: the complete integration of the technology that is embedded inside anticorrosion coating systems without any aesthetic or aerodynamic impact; the thinner embedded strain gauges comparison to OFSs may be less invasive; simple and direct data interpretation; and probably an easier and less expensive fabrication process. On the contrary, if these hypotheses were ever confirmed, a thorough study of the coating to be monitored would have to be conducted in a lab in order to understand its weathering response and degradation mechanisms. Moreover, despite relatively similar trends, degradation modes are unique for each coating type, which makes it imperative to study each coating system beforehand. Finally, an offset correction would also be necessary, as temperature greatly influences the sensors [105][52].References
- Han, Y.; Wang, J.; Zhang, H.; Zhao, S.; Ma, Q.; Wang, Z. Electrochemical impedance spectroscopy (EIS): An efficiency method to monitor resin curing processes. Sens. Actuators A Phys. 2016, 250, 78–86.
- Miszczyk, A.; Miszczyk, A.; Schauer, T. Electrochemical approach to evaluate the interlayer adhesion of organic coatings Article in Progress in Organic Coatings · April 2005 Tworzywa konstrukcyjne odporne na gorące kwasy mineralne 1990 View project Chemometrics methods in corrosion View project. Prog. Org. Coat. 2005, 52, 298–305.
- Moreno, C.; Hernández, S.; Santana, J.J.; González-Guzmán, J.; Souto, R.M.; González, S. Characterization of water uptake by organic coatings used for the corrosion protection of steel as determined from capacitance measurements. Int. J. Electrochem. Sci. 2012, 7, 7390–7403.
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Determination of water uptake and diffusion of Cl-ion in epoxy primer on aluminum alloys in NaCl solution by electrochemical impedance spectroscopy. Prog. Org. Coat. 2003, 46, 273–279.
- Duarte, R.G.; Castela, A.S.; Ferreira, M.G.S. A new model for estimation of water uptake of an organic coating by EIS: The tortuosity pore model. Prog. Org. Coat. 2009, 65, 197–205.
- Nguyen, A.S.; Causse, N.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Determination of water uptake in organic coatings deposited on 2024 aluminium alloy: Comparison between impedance measurements and gravimetry. Prog. Org. Coat. 2017, 112, 93–100.
- Deflorian, F.; Fedrizzi, L. Adhesion characterization of protective organic coatings by electrochemical impedance spectroscopy. J. Adhes. Sci. Technol. 1999, 13, 629–645.
- Hinton, A.J. Determination of coating adhesion using electrochemical impedance spectroscopy. Solartron Anal. 2010, 2, 18–23.
- Hu, J.; Li, X.; Gao, J.; Zhao, Q. UV aging characterization of epoxy varnish coated steel upon exposure to artificial weathering environment. Mater. Des. 2009, 30, 1542–1547.
- Kittel, J.; Celati, N.; Keddam, M.; Takenouti, H. New methods for the study of organic coatings by EIS: New insights into attached and free films. Prog. Org. Coat. 2001, 41, 93–98.
- Armas, R.A.; Gervasi, C.A.; Di Sarli, A.; Real, S.G.; Vilche, J.R. Zinc-rich paints on steels in artificial seawater by electrochemical impedance spectroscopy. Corrosion 1992, 48, 379–383.
- Beiro, M.; Collazo, A.; Izquierdo, M.; Nóvoa, X.R.; Pérez, C. Characterisation of barrier properties of organic paints: The zinc phosphate effectiveness. Prog. Org. Coat. 2003, 46, 97–106.
- Bierwagen, G.; Tallman, D.; Li, J.; He, L.; Jeffcoate, C. EIS studies of coated metals in accelerated exposure. Prog. Org. Coat. 2003, 46, 149–158.
- Ribeiro, D.V.; Souza, C.A.C.; Abrantes, J.C.C. Use of Electrochemical Impedance Spectroscopy (EIS) to monitoring the corrosion of reinforced concrete. Rev. IBRACON Estrut. Mater. 2015, 8, 529–546.
- Gamry, I. Basics of Electrochemical Impedance Spectroscopy. Available online: https://www.gamry.com/assets/Application-Notes/Basics-of-EIS.pdf (accessed on 1 April 2022).
- Allahar, K.N.; Bierwagen, G.; Battocchi, D.; Gelling, V.J. Examination of the feasibility of the use of in-situ corrosion sensors in army vehicules. In Proceedings of the Tri-Service Corrosion Conference, Houston, TX, USA, 14–18 November 2005.
- Brasher, D.M.; Kingsbury, A.H. Electrical measurements in the study of immersed paint coatings on metal. I. Comparison between capacitance and gravimetric methods of estimating water-uptake. J. Appl. Chem. 1954, 4, 62–72.
- Le Thu, Q.; Takenouti, H.; Touzain, S. EIS characterization of thick flawed organic coatings aged under cathodic protection in seawater. Electrochim. Acta 2006, 51, 2491–2502.
- Amirudin, A.; Thieny, D. Application of electrochemical impedance spectroscopy to study the degradation of polymer-coated metals. Prog. Org. Coat. 1995, 26, 1–28.
- Bierwagen, G.; Wang, X.; Tallman, D. In situ study of coatings using embedded electrodes for ENM measurements. Prog. Org. Coat. 2003, 46, 163–175.
- Merten, B.E.; Battocchi, D.; Tallman, D.E.; Bierwagen, G.P. Embedded Reference Electrode for Potential-Monitoring of Cathodic Protective Systems. J. Electrochem. Soc. 2010, 157, C244.
- Davis, G. EIS-based in-situ sensor for the early detection of coating degradation and substrate corrosion. In Proceedings of the CORROSION 2000, Orlando, FL, USA, 26–31 March 2000.
- Davis, G.D.; Ross, R.A.; Raghu, S.D. Coating health monitoring system for army ground vehicles. In Proceedings of the CORROSION 2007, Nashville, TN, USA, 11–15 March 2007.
- Kittel, J.; Celati, N.; Keddam, M.; Takenouti, H. Influence of the coating-substrate interactions on the corrosion protection: Characterisation by impedance spectroscopy of the inner and outer parts of a coating. Prog. Org. Coat. 2003, 46, 135–147.
- Duarte, R.G.; Castela, A.S.; Ferreira, M.G.S. Influence of the solution cation mobility on the water uptake estimation of PVC Plastisol freestanding films by EIS. Prog. Org. Coat. 2006, 57, 408–415.
- Bierwagen, G.P.; Allahar, K.N.; Su, Q.; Gelling, V.J. Electrochemically characterizing the ac–dc–ac accelerated test method using embedded electrodes. Corros. Sci. 2009, 51, 95–101.
- Allahar, K.; Su, Q.; Bierwagen, G. Non-substrate EIS monitoring of organic coatings with embedded electrodes. Prog. Org. Coat. 2010, 67, 180–187.
- Upadhyay, V.; Allahar, K.N.; Bierwagen, G.P. Environmental humidity influence on a topcoat/Mg-rich primer system with embedded electrodes. Sens. Actuators B Chem. 2014, 193, 522–529.
- Brossia, C.S. Apparatus and Method for Detecting the Degradation of a Coating Using Embedded Sensors. U.S. Patent 6,911,828B1, 23 May 2005.
- De Rosa, R.L.; Earl, D.A.; Bierwagen, G.P. Statistical evaluation of EIS and ENM data collected for monitoring corrosion barrier properties of organic coatings on Al-2024-T3. Corros. Sci. 2002, 44, 1607–1620.
- Iverson, W.P. Transient Voltage Changes Produced in Corroding Metals and Alloys. J. Electrochem. Soc. 1968, 115, 617.
- Skerry, B.S.; Eden, D.A. Characterisation of coatings performance using electrochemical noise analysis. Prog. Org. Coat. 1991, 19, 379–396.
- Bierwagen, G.P. Calculation of Noise Resistance from Simultaneous Electrochemical Voltage and Current Noise Data. J. Electrochem. Soc. 1994, 141, L155.
- Cottis, R.A. Electrochemical noise for corrosion monitoring. In Techniques for Corrosion Monitoring; Woodhead Publishing Series in Metals and Surface Engineering; Elsevier: Cambridge, UK, 2021; pp. 99–122.
- Bertocci, U. Noise Resistance Applied to Corrosion Measurements. J. Electrochem. Soc. 1997, 144, 37.
- Jamali, S.; Mills, D.J.; Woodcock, C. Ways of increasing the effectiveness of the electrochemical noise method for assessment of organic coatings on metal. ECS Trans. 2010, 24, 115–125.
- Rodriguez-Pardo, L.; Cao-Paz, A.; Fariña, J.; Covelo, A.; Nóvoa, X.R.; Pérez, C. Water uptake kinetics in anti-corrosion organic films with a high resolution microbalance oscillator sensor. Sens. Actuators B Chem. 2010, 144, 443–449.
- Mabbutt, S.J.; Mills, D.J. Technical note Novel configurations for electrochemical noise measurements. Br. Corros. J. 1998, 33, 158–160.
- Mabbutt, S.; Mills, D.J.; Woodcock, C.P. Developments of the electrochemical noise method (ENM) for more practical assessment of anti-corrosion coatings. Prog. Org. Coat. 2007, 59, 192–196.
- Mills, D.J.; Broster, M.; Razaq, I. Continuing work to enable electrochemical methods to be used to monitor the performance of organic coatings in the field. Prog. Org. Coat. 2008, 63, 267–271.
- Su, Q.; Allahar, K.; Bierwagen, G. Embedded electrode electrochemical noise monitoring of the corrosion beneath organic coatings induced by ac–dc–ac conditions. Electrochim. Acta 2008, 53, 2825–2830.
- Allahar, K.N.; Upadhyay, V.; Bierwagen, G.P.; Gelling, V.J. Monitoring of a military vehicle coating under prohesion exposure by embedded sensors. Prog. Org. Coat. 2009, 65, 142–151.
- Telegdi, J.; Shaban, A.; Vastag, G. Biocorrosion—Steel. In Encyclopedia of Interfacial Chemistry; Elsevier: New York, NY, USA, 2018; pp. 28–42.
- Rahman, S.U.; Atta Ogwu, A. Corrosion and Mott-Schottky probe of chromium nitride coatings exposed to saline solution for engineering and biomedical applications. In Advances in Medical and Surgical Engineering; Elsevier: London, UK, 2020; pp. 239–265.
- Perrin, F.X.; Merlatti, C.; Aragon, E.; Margaillan, A. Degradation study of polymer coating: Improvement in coating weatherability testing and coating failure prediction. Prog. Org. Coat. 2009, 64, 466–473.
- Shin, C.-S.; Liaw, S.-K.; Yang, S.-W. Post-Impact Fatigue Damage Monitoring Using Fiber Bragg Grating Sensors. Sensors 2014, 14, 4144–4153.
- Ramezani-Dana, H.; Casari, P.; Perronnet, A.; Fréour, S.; Jacquemin, F.; Lupi, C. Hygroscopic strain measurement by fibre Bragg gratings sensors in organic matrix composites - Application to monitoring of a composite structure. Compos. Part B Eng. 2014, 58, 76–82.
- Marro Bellot, C.; Olivero, M.; Sangermano, M.; Salvo, M. Towards self-diagnosis composites: Detection of moisture diffusion through epoxy by embedded evanescent wave optical fibre sensors. Polym. Test. 2018, 71, 248–254.
- Enser, H.; Sell, J.K.; Hilber, W.; Jakoby, B. Printed strain sensors in organic coatings: In depth analysis of sensor signal effects. Sens. Actuators A Phys. 2018, 281, 258–263.
- Enser, H.; Kulha, P.; Sell, J.K.; Schatzl-Linder, M.; Strauß, B.; Hilber, W.; Jakoby, B. Printed strain gauges embedded in organic coatings—Analysis of gauge factor and temperature dependence. Sens. Actuators A Phys. 2018, 276, 137–143.
- Enser, H.; Kulha, P.; Sell, J.K.; Jakoby, B.; Hilber, W.; Strauß, B.; Schatzl-Linder, M. Printed Strain Gauges Embedded in Organic Coatings. Procedia Eng. 2016, 168, 822–825.
- Kulha, P.; Enser, H.; Sell, J.K.; Strauß, B.; Schatzl-Linder, M.; Jakoby, B.; Hilber, W. Temperature dependence of gauge factor of printed piezoresistive layers embedded in organic coatings. Proceedings 2017, 1, 618.
- Zhang, Y.; Anderson, N.; Bland, S.; Nutt, S.; Jursich, G.; Joshi, S. All-printed strain sensors: Building blocks of the aircraft structural health monitoring system. Sens. Actuators A Phys. 2017, 253, 165–172.
- Zymelka, D.; Togashi, K.; Ohigashi, R.; Yamashita, T.; Takamatsu, S.; Itoh, T.; Kobayashi, T. Printed strain sensor array for application to structural health monitoring. Smart Mater. Struct. 2017, 26, 105040.
- Sell, J.K.; Enser, H.; Jakoby, B.; Schatzl-Linder, M.; Strauss, B.; Hilber, W. Printed Embedded Transducers: Capacitive Touch Sensors Integrated into the Organic Coating of Metalic Substrates. IEEE Sens. J. 2016, 16, 7101–7108.
