1. Introduction
Caprine farming has spread to almost every country in the world, due to the good prices and high value of goat-derived products (especially milk), attracting new farmers and investors
[1]. The majority of the world caprine population is located in developing countries, occupying marginal territories under extreme climate conditions and held under elder farming systems
[2]. This scene contrasts with that of Europe and North America, where otherwise, high-technological and intensive conditions rule the goat industry, which is highly focused on milk production and exploiting high-performance breeds subjected to genetic selection schemes
[3]. In this context, the benefits that the domestic goat has obtained, as derived from the achievements made in the areas of genetics, nutrition and animal management, are rather limited in comparison to the level of integration that such techniques account for in other species.
The aforementioned framework evidences the secondary role to which caprine has been relegated within the scope of stockbreeding history
[4]. This secondary position may be the result of two main conjoined facts; the traditional disregard of the caprine species as a destructive animal for pasture
[5], and consumer preferences for other domestic species, which have conferred caprine-derived products with a low international market value, thus pushing caprine production to a marginal role in farming
[6].
Recent archaeological findings indicate that the domestication of the goat took place more than 10,000 years ago in the ‘Fertile Crescent’ (
Figure 1). This region is where the first settled agricultural communities of the Middle East and Mediterranean basin are thought to have originated, and would have covered the area from the Anatolian peninsula to the eastern territories of current Iran
[7]. Wild bezoar (
Capra aegagrus) and markhor (
Capra falconeri) are thought to be the most likely ancestors of the domestic goat, according to phylogenetic studies implementing Y chromosome AMELY and ZFY sequences
[8]. Additionally, other studies evaluating the major histocompatibility complex led to the possible inclusion of the Iberian mountain goat (
Capra pyrenaica) and Alpine ibex (
Capra ibex) in the history of goat domestication
[3].
Figure 1. Relief Representation of Goatherd with Goat and Trees, ca. 1350–1333 BCE. New Kingdom, Amarna Period. Late Dynasty 18. Limestone, 8 1/4 × 16 3/4 × 2 1/2 in., 22.5 lb. (21 × 42.5 × 6.4 cm, 10.21 kg). Brooklyn Museum, Gift of the Ernest Erickson Foundation, Inc., New York, NY, USA, 86.226.30.
A higher tolerance to human handling and a better adaptability to the driven grazing may have been determining factors, which aimed to boost the popularity of certain animal populations
[9]. Apart from the early breeding objectives that were sought following the domestication of the goat, the first civilizations became interested in the functional selection objectives linked to productive traits, such as the aptitude to captive breeding, prolificacy or body size
[10].
The domestication and world dissemination of the species led to the first distinctive morphological traits of the original goat populations, such as the shape of the horns and ears
[3]. This source of phenotypic variability could be the result of human/artificial selection, in addition to genetic drift and founder effects
[11], which may explain the appearance of the characteristic traits in a lineage as a consequence of a narrow genetic base in its original population and its isolation. This may also be evidenced by other features, such as the presence of wattles, hair length or the wide variety of possible coat colours that have been developing as other distinctive traits in the first goat populations
[3].
After centuries of their relationship with humans, natural selection for caprine adaptability to different environments and the artificial selection for productive, morphological and behaviour traits led to the appearance of 576 modern domestic goat breeds
[10]. The Angora goat, whose presence in Phrygia and Cilicia (current Anatolia peninsula) was described 2400 years BC
[12], was the first caprine breed in which a preliminary process of standardization was attempted. However, it would not be until 1890 and 1895 when the first caprine dairy goat breed standardization would take place in Switzerland, for the Saanen and Toggenburg goat breeds, respectively
[13].
Although this event was a milestone and marked the beginning of caprine milk selection history
[13], most of the significant advances would have to wait until the 1960s in France, when a bovine selection model would be applied to dairy goat production
[14]. This turning point was promoted by the massive growth in the French cheese industry after the Second World War, which led to the development of intensive milking farm regions connected to the cheese factories. This improvement in animal production required farms to implement highly technical support, which brought about the routinization of progeny testing and artificial insemination
[13]. It would take another decade for caprine meat breeding programmes (which have been dramatically scarcer than dairy goat breeding programs) to appear, with Boer goat breeding programmes being one of the few examples, appearing by the end of the 1970s in South Africa
[15] (
Figure 2).
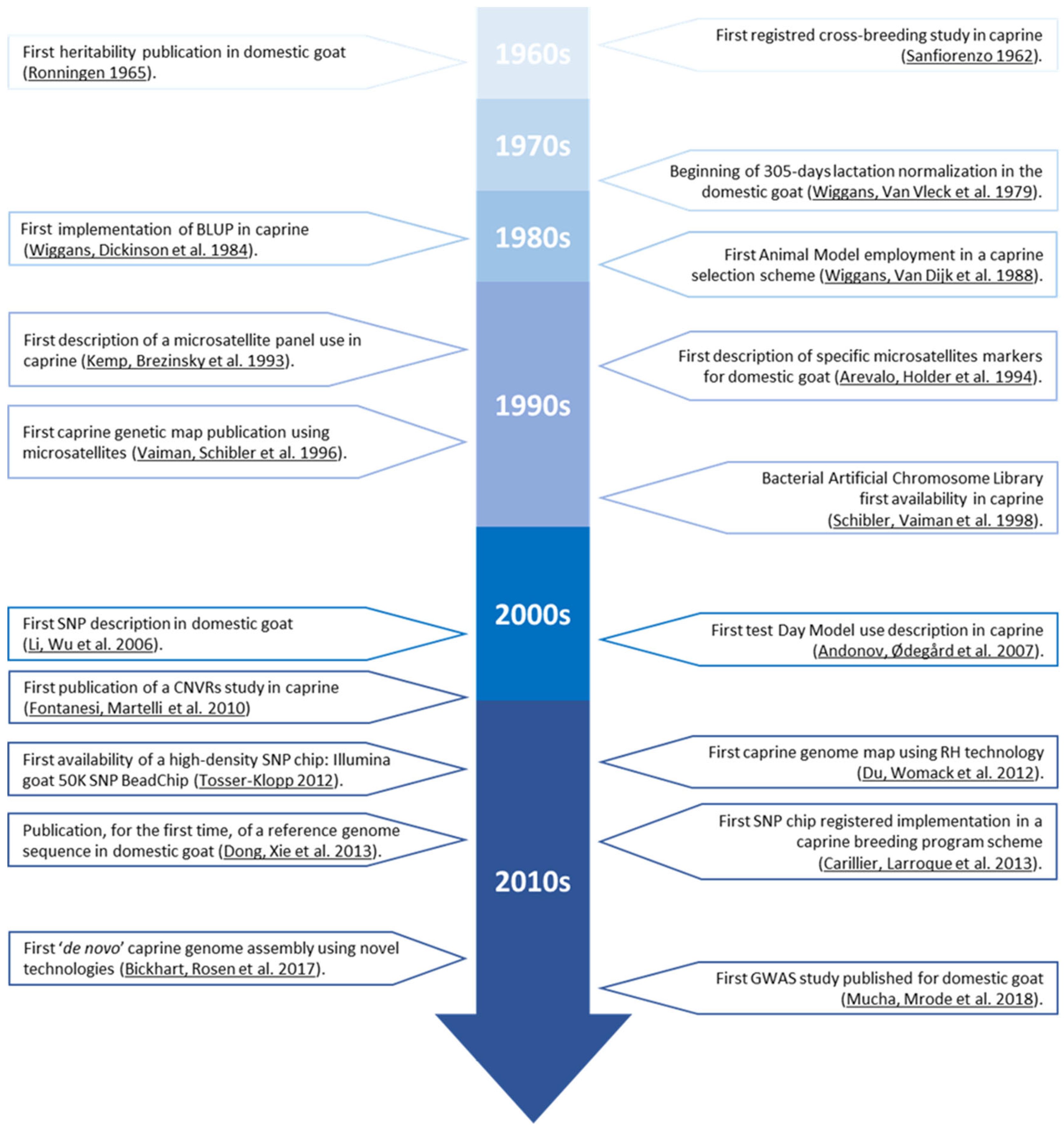
The first documented register describing a caprine-selection-focused attempt dates back to 1962
[16], when the ‘Universidad de Puerto Rico’ studied the most profitable crosses between Puerto Rican local goat breeds and high-performance dairy goats. The study sought the most appropriate cross that would result in animals parallelly presenting the best adaptability, through the evaluation of goat kid survival rate, and the greatest productivity, through the evaluation of milk yield.
It was three years later (1965) when the first heritability estimations were performed in caprine
[17]. This advance resulted from the integration of genealogical information as a compulsory step towards animal breeding estimations, which is a crucial step during the first stages of any breeding programme. The advances not only concerned the available genetic components or biostatistical tools, but also the revolution of phenotypic data collection. Contextually, the onset of 305-day lactation normalization in 1979
[18] made it possible to objectively compare the productivity of does from different breeds and controlled at different moments within lactation
[19], which not only permitted farmers’ taking directed decisions based on factual data, but also laid the grounds for genetic evaluations. Still, the control of environmental effects was challenging, and reliable estimations were not feasible.
After the implementation of Best Linear Unbiased Prediction methods (BLUP) in caprine in the mid-1980s
[20], along with the Animal Model
[21] for breeding value calculations, the design of more complex selection schemes arose. This permitted the complete and reliable integration of genealogical information into genetic evaluations, but also the evaluation of animals that were phenotypically controlled in broadly distinct environmental conditions. This means that BLUP allowed for the separate estimation of the genetic and non-genetic (environmental) factors; hence, the heritable fraction of functional traits could be isolated and more appropriately controlled.
The ‘Genomic Era’ began with the discovery and utilization of microsatellite markers, which would be applied in the context of domestic goats as an extension of the use of bovine and ovine microsatellite panels in 1993
[22]. One year later, in 1994, the first description of caprine specific microsatellites was published
[24]. The genomic information obtained from caprine microsatellite studies in these years permitted the development of studies based on the relationship between specific genomic regions, Quantitative Trait Loci (QTL), and desirable production traits
[19].
QTLs are regions of the genome for which an association with the phenotypic variance of a certain trait has been proved
[34]. Such an association may be supported by the fact that QTL regions may contain genes codifying for the specific regulation of the expression of a certain functional feature. For several years, many QTL were described using microsatellite genetic markers. However, even if they are still valid and preferrable when economic resources for research are scarce, the large size of some QTL
[34] makes their mapping resolution and confidence intervals limited if the application of other, more efficient techniques is possible
[35].
As previously mentioned, despite the fact that microsatellites offer a high degree of polymorphism for each marker, they are not as abundant as SNPs, and hence provide insufficient coverage of the genome
[36]. In this regard, the first study of a caprine Single Nucleotide Polymorphism (SNP) was published in 2006
[37]. SNP would progressively replace microsatellites as the preferred genetic marker.
Additional milestones were quickly reached in the following years. For instance, the first study using the Canadian Test-Day Model in caprine was published in 2007
[38]. The Canadian Test-Day Model is a 12-trait random regression animal model, in which the traits are milk, fat, and protein test-day yields, and somatic cell scores on test days within each of the first three lactations. Test-day records from later lactations are not used. Random regressions (genetic and permanent environmental) are based on Wilmink’s three parameter function, which includes an intercept, regression on days in milk, and regression on an exponential function to the power –0.05 times days in milk (b0 + b1 × Exp(−0.05 × days in milk) + b2 × days in milk)
[39]. This translates into genetic evaluations based on a better modelling of the lactation curve, providing more accurate results, which consequently enhances the selection progress.
These advances led to the publication of the first ‘Copy Number Variations Regions’ (CNVR) map for the domestic goat in 2010
[31]. Recent studies have shown that CNVR (intraspecific gains or losses of ≥1 kb of genomic DNA), represent important sources of variability of mammalian genomes (~0.4–25% of the genome). Their importance lies in the fact that CNVRs can change the gene structure and dosage, regulate gene expression and function and, hence, potentially have more effects than the most frequent single-nucleotide polymorphisms (SNPs) in determining phenotypic differences.
In 2012, the ‘International Goat Genome Consortium’ (IGGC) developed the first SNP chip for domestic goats; a high-density chip with 53,347 SNPs called ‘
Illumina 50K SNP BeadChip’ (Illumina Inc., San Diego, CA, USA)
[30]. In 2014, a new version of the high density SNP chip was developed, which is the most advanced goat SNP chip at present, called the ‘
Illumina 52K SNP BeadChip’ a 60,000 SNP chip (Illumina Inc., San Diego, CA, USA), which is also carried out under the support of the IGGC
[40]. Due to its robustness, low genotyping costs, automatic allele calling and the ability to interrogate the goat genome at high resolutions, these SNP chips were used to study the genetic diversity and population structure of native goats in various countries
[41].
In 2013, after several attempts
[23][25][42], the whole caprine genome was sequenced and optically mapped in China
[29]. This advance made the reference genome sequence of the domestic goat available for the first time. The knowledge of the caprine genomic map was preceded by the first ‘Bacterial Artificial Chromosome Library’ published for caprine
[43]. Recently, a new, ‘de novo’ genetic assembly (ARS1) has been developed. It allows for high-quality genomic mapping, thanks to the advanced sequencing methodology ‘
single-molecule PacBioRSII’
[27]. This methodology uses single-molecule, real-time (SMRT) sequencing technology, which takes advantage of an immobilized DNA polymerase/template complex nested in thousands of small wells (called zero-mode waveguides). The value of these new technologies lies in the fact that they are characterized by their high-throughput characteristics. Hence, they provide the opportunity to produce millions of reads with an inexpensive sequencing.
2. Candidate Genes Regulating the Expression of Economically Relevant Traits in Caprine
2.1. Candidate Genes Regulating the Expression of Goat Meat Breeding Criteria and Traits
Growth performance and weight gain are two of the most relevant traits in caprine farming, given that they directly relate to the shortening of puberty age and the number of kilograms of meat to be sold, hence benefitting the farmer
[44]. Contextually, body weight increases until 5–6 years of age, at which time it begins to decrease. The literature indicates the existence of sexual dimorphism, with males being heavier than females and a progressive decrease in weight gain as the number of kids born increases, with single-birth kids being heavier than double-birth kids, and these, in turn, being heavier than multiple-birth kids. Among other important factors, the season of birth, nutrition management, farming system, and the age and birth order of the doe have been reported to influence body-weight-related traits
[45].
The current selection framework is characterized by a lack of reciprocity between caprine dairy and meat production. Hence, in practice, this is one of the main challenges that the sector needs to face; improving dairy characteristics, which negatively correlate with those related to butchering production
[46], while taking advantage of the sturdiness and improved adaptability of caprine breeds
[47].
The breeding objectives used in artificial selection to obtain a desirable dairy morphology are the opposite to those seeking the enhancement of meat/butcher traits. This may explain the marked differences that exist between the characteristic shape of goat dairy breeds (thin and triangular forms), and meat breeds (short, rectangular and excellent body conformation)
[44].
Carcass dressing percentage in goats is approximately 50–55%, and is usually lower than in ovine. This is due to the greater bone proportion in caprine, along with a different carcass fat deposition, which, in goats, is perivisceral rather than subcutaneous or intramuscular
[48]. In the same way, the caprine carcass has a lower fat coverage; hence, moisture losses (which can reach 8%) are greater than those in sheep. However, the lower intramuscular fat deposition in caprine makes chevon meat a healthier alternative to lamb
[49].
In terms of meat quality characteristics, organoleptic traits include muscle appearance, juiciness, texture, hardness, flavour and aroma
[50]. Many factors condition the expression of these traits, including nutrition, exercise/physical activity, age, and method of slaughter and bleeding
[50]. Regarding nutritional quality, chevon muscle is high in amino acid diversity and is very lean, presenting a lower degree of fat interspersion than other species (but with more polyunsaturated fatty acids)
[6].
Body weight and butcher performance, body growth, body weight gain, kid survival rate, feed conversion index and dressing percentage of the carcass are among the most frequently considered quantitative traits within the framework of caprine selection.
Table 1 presents a summary of the most frequently addressed breeding criteria and traits concerning selection for meat production in goats.
Figure 3 presents a scheme of caprine candidate gene background regulating the expression of meat production.
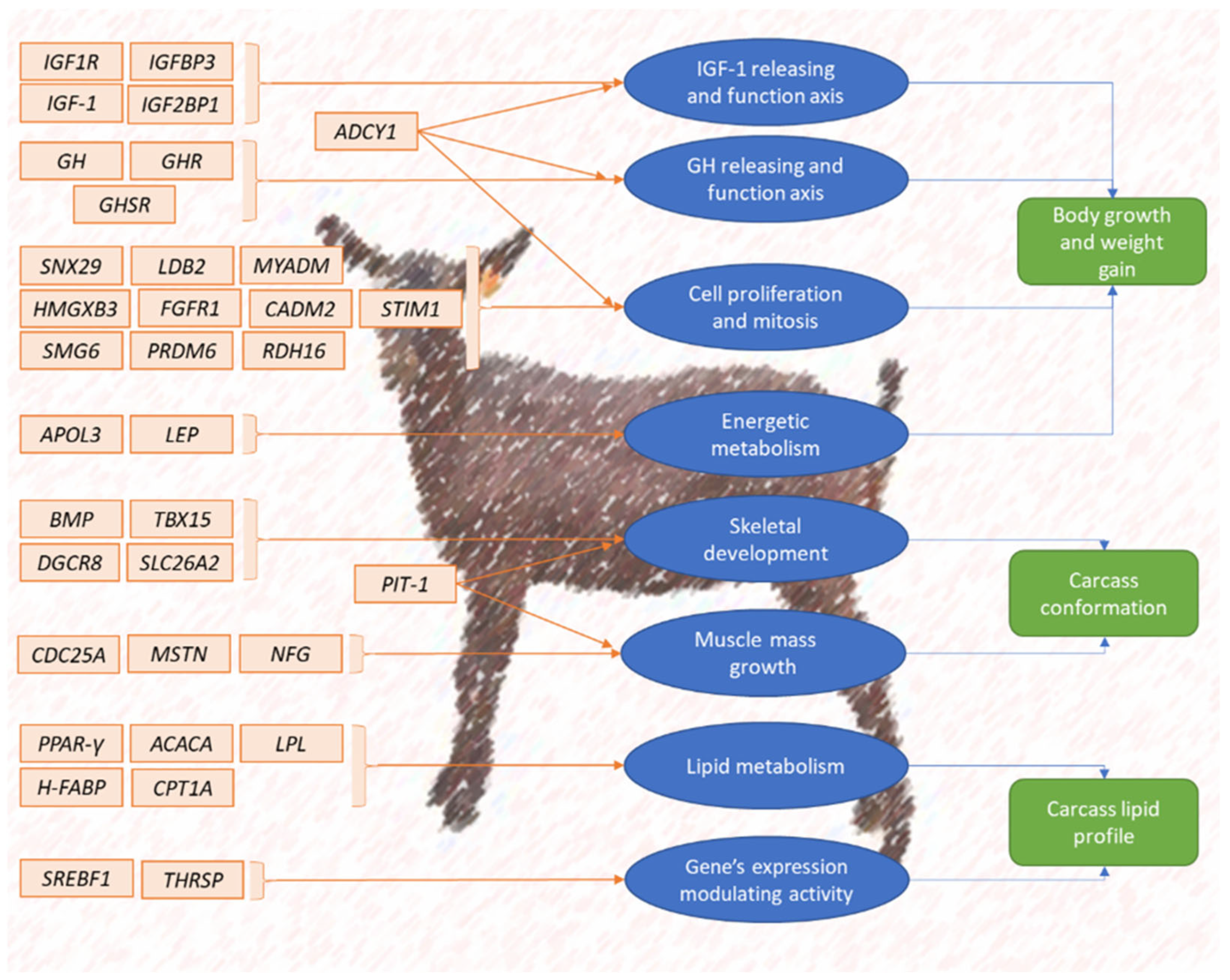
Figure 3. Summary scheme of caprine candidate genes’ influence on meat production.
Table 1. Breeding criteria and traits for caprine meat production.
|
Breeding Criteria
|
Traits
|
Reference
|
|
Body weight
|
Birth weight, body weight at 7, 14, 21 and 28 days, monthly weighing until 18 months of age
|
[51][52]
|
|
Growth
|
Average daily gain (ADG) before weaning, from 3 to 6 months of age, from 6 to 12 months of age
|
[52]
|
|
Conformation/corporal structure
|
Body length, height at the withers, chest girth, shoulder width and pin-bone width
|
[53]
|
|
Carcass quality
|
Hot (immediately after slaughter) and cold (before chilling) carcass weight; weights of the head, skin, heart, and thoracic and abdominal viscera; carcass dressing percentage; leg length and carcass width; and carcass fat coverage and lean meat and bone yield percentages
|
[51][54]
|
|
Meat quality
|
Muscle pH measurements, colour, water retention capacity, nutritional composition (protein, fat, and collagen percentages), and fatty acid profile in samples of longissimus thoracis et lumborum, triceps brachii and semimembranosus
|
[51]
|
2.2. Candidate Genes Regulating the Expression of Goat Dairy Breeding Criteria and Traits
The international caprine milking sector comprised 215 million animals in 2019, which was one-quarter of the world caprine census
[55]. Europe, with only 5% of the world caprine dairy census, produced 15% of the caprine worldwide milk production, which could mainly be ascribed to its high degree of specialization
[1].
This high European productivity is supported on high-technology farms, on which goat breeds such as the Alpine, Saanen or Toggenburg have been bred under the scope of strict genetic selection schemes and breeding programmes
[3]. However, this is only one side of the same coin and the opposite situation is found in developing countries. Specifically, in developing countries, genealogic control and productivity registers are barely available or are of poor quality
[1]. The caprine European dairy sector is a well-regulated industry, where almost all the milk is processed into cheese
[1]. Most of the farms’ income derives from milk production, while the sale of chevon for the meat industry is a marginal source of income, due to the relatively low value of caprine meat within developed countries
[6].
According to García, et al.
[56], milk quality is defined as the milk’s ability to tolerate the technological processes that lead to a market-demanded product in terms of nutritional value, food safety and sensorial parameters. This is especially relevant in the framework of the cheese industry, given that increasing milk protein and percentage (known as ‘dry’ or ‘cheese extract’) increases the chances to enhance farms’ rentability and profitability, even more than milk volume production (in litres)
[57]. Furthermore, traditional breeding criteria (milk yield in volume; milk lactose, fat and protein percentages; fatty acid profile and omega 3 (technologic quality); and somatic cell count (hygienic quality)
[56]) have recently been joined by postprocessing technological traits related to
cheese-making quality indicators, in order to improve cheese production efficiency. Among these traits the researchers find, for instance, milk cheese yield (%CY) or the dairy cheese efficiency (dCY)
[57], and the newly implemented term of ‘recuperation’ (%REC) for each milk component (proteins and fat) from the junket.