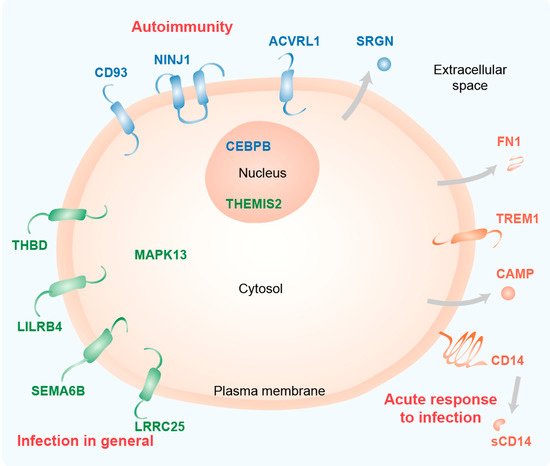
Figure 15. Functional profile of key immune-related vitamin D target genes. Schematic picture of a cell indicating the main location of the proteins encoded by the 15 key genes. The information is based on GeneCards (
www.genecards.org) and publications cited in the text. The classification of the proteins (group 1: orange, group 2: green, group 3: blue) is based on their transcriptome profile. The main immune-related function of the protein groups is indicated in red.
The majorly increased production of the anti-microbial peptide CAMP is a well-known example of the action of vitamin D in supporting innate immunity in the fight against bacteria, such as Mycobacterium tuberculosis
[29][30]. The glycoprotein CD14 shows highest expression in monocytes and macrophages. It is anchored via glycosylphosphatidylinositol on the surface of the plasma membrane and is also found in a secreted form (sCD14). CD14 acts as a co-receptor for the pattern recognition receptors TLR1-4, 6, 7 and 9
[31]. It delivers the pathogen-associated molecule lipopolysaccharide (LPS), which is produced exclusively by gram-negative bacteria, to TLR4 resulting in pro-inflammatory responses
[32]. The transmembrane glycoprotein TREM1, which is found in monocytes, macrophages and neutrophils, is also heavily involved in TLR4 signaling, i.e., it promotes inflammation in response to bacterial infection
[33]. TREM1 is already known as a vitamin D target gene
[34]. Similarly, the gene encoding for the extracellular matrix protein FN1 has long been known as a vitamin D target
[35]. The protein is secreted by a large variety of cell types, such as macrophages, fibroblasts and epithelial cells. FN1 functions in cell adhesion and wound healing but also participates in LPS/TLR4 signaling, i.e., in the inflammatory responses
[36]. Thus, the proteins encoded by the highly vitamin D-responsive genes of group 1 are all involved in acute responses to infection.
THBD is a transmembrane protein expressed in endothelial cells, monocytes and macrophages
[37]. The traditional role of THBD is to bind thrombin and turn its pro-coagulative action to anti-coagulative, i.e., reducing blood clots. However, THBD can also bind LPS and induces its binding to the complex of CD14 and TLR4, i.e., it prevents the pro-inflammatory consequences of NF-ĸB signaling
[38]. The THBD gene is known as a key vitamin D target both in monocytes
[39] and in PBMCs
[40]. LILRB4 is an inhibitory immuno-regulatory receptor that acts on antigen-presenting cells like dendritic cells, macrophages, monocytes and microglia. It affects TNF production and bactericidal activity
[41] as well as the modulation of the differentiation of regulatory T cells
[42]. The LILRB4 gene is known as a vitamin D target gene in PBMCs
[43]. The transmembrane protein SEMA6B belongs to a the semaphorin protein family, members of which have immune functions related to the control of cell movements and cell-cell communication
[44]. Interestingly, the family member SEMA3B is known as a vitamin D target gene in bone
[45]. The transmembrane protein LRRC25 is found in monocytes, dendritic cells, granulocytes and T lymphocytes. It acts as a negative regulator of the signaling pathways of NF-ĸB
[46] and interferon
[47], i.e., it suppresses the production of inflammatory cytokines and modulates the response to viral infections. The latter is a long overlooked effect of vitamin D on the immune system
[48][49] and a reason why vitamin D deficiency may lead to high vulnerability against viral infections in the elderly, but also in school children
[15][50][51].
The kinase MAPK13 is involved in LPS/TLR4 signaling and together with MAPK11 it regulates cytokine-induced inflammatory responses
[52]. The MAPK13 gene is expressed in a large variety of cell types and it has already been described as a vitamin D target in skeletal muscle
[53]. The TLR signal transduction modulatory protein THEMIS2 is expressed in B lymphocytes, macrophages and dendritic cells and regulates LPS-induced TNF production downstream of TLR4
[54]. Moreover, the protein is involved in the development of T lymphocytes and serves as a marker of monocytic differentiation
[55]. Thus, the proteins encoded by the mid vitamin D responsive genes of group 2 are primarily involved in general responses to infection.
CD93 is a transmembrane glycoprotein that is expressed primarily in endothelial cells but also in granulocytes, monocytes, platelets and stem cells
[56]. The protein is involved in several processes of innate immunity, such as adhesion, phagocytosis and inflammation
[57]. In the context of the latter, CD93 may act as a plasma membrane receptor for DNA for delivery to endosomal TLR9. Moreover, CD93 boosts the inflammatory response of monocytes by increasing LPS recognition by TLR4. CD93 may have a protective role in autoimmune encephalomyelitis via the control of the severity of inflammation, apoptosis and bystander neuronal injury
[58]. NINJ1 is a transmembrane protein expressed primarily in myeloid and endothelial cells
[59] but it was first described in the peripheral nervous system inducing neurite extension. In the latter context, NINJ1 was found to be involved in the immune pathogenesis of multiple sclerosis
[60]. NINJ1 functions in cell adhesion and inflammation, such as leukocyte migration to sites of inflammation in the endothelium. CEBPB is a transcription factor that plays diverse roles in inflammation, e.g., through T helper cell 17 (TH17)-dependent regulation of inflammation in models of multiple sclerosis
[61]. The
CEBPB gene has already been described as a vitamin D target in myeloid leukemia cells
[62]. ACVRL1 is a transmembrane protein of the transforming growth factor beta superfamily, which mediates the bone morphogenetic protein (BMP) 9- and BMP10-induced signaling that orchestrates the development of blood vessels
[63]. This relates to the control of monocyte to macrophage differentiation
[64].
SRGN is a secreted proteoglycan of endothelial cells, monocytes, mast cells and lymphocytes
[65]. LPS up-regulates SRGN expression in macrophages, suggesting the protein plays a role of in storage and secretion of inflammatory mediators
[66]. Thus, the proteins encoded by the low vitamin D responsive genes of group 3 are primarily involved in autoimmunity.