Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Maria José Pérez Alvarez and Version 2 by Conner Chen.
ammalian/mechanistic target of rapamycin (mTOR) is a 289 kDa serine–threonine kinase and a key element of two mTOR complexes called mTORC1 and mTORC2 (mTORCs). Furthermore, mTOR is highly conserved and is the center of multiples signaling pathways and coordinates important cellular processes such as cell growth and metabolism. Although mTOR is ubiquitously expressed, it is especially abundant in the brain. Therefore, mTOR dysfunction profoundly affects the central nervous system (CNS).
- mTOR
- Hypoxia
- Ischemia
- Brain
- Neuron
1. The Structure of mTOR and Its Complexes in the Brain
Mammalian/mechanistic target of rapamycin (mTOR) is a 289 kDa serine–threonine kinase and a key element of two mTOR complexes called mTORC1 and mTORC2 (mTORCs) [1][2][3][4][1,2,3,4]. Furthermore, mTOR is highly conserved and is the center of multiples signaling pathways and coordinates important cellular processes such as cell growth and metabolism [5]. Although mTOR is ubiquitously expressed, it is especially abundant in the brain [6]. Therefore, mTOR dysfunction profoundly affects the central nervous system (CNS). Mutations in genes encoding mTOR regulators induce neurological disorders called “mTORopathies” [5].
Furthermore, mTOR, as indicated by its name, is a target protein of rapamycin, an immunosuppressant and anti-fungal macrolide compound isolated from Streptomyces hygroscopicus. This kinase comprises several functional domains, including C-terminal small FAT domain (FATC), C-terminal kinase domain (KD), FKBP12 rapamycin-binding domain (FRB), transactivation/transformation-associated domain (FAT), and an N-terminal domain containing at least 20 HEAT (Huntingtin elongation factor 3 A subunit of PP2A TOR1) repeats. The latter provide sites for the interaction of regulatory proteins to form mTORC1 and mTORC2. The KD domain of mTOR, with conserved sequences homologous to the catalytic domain of the phosphoinositide 3-kinase (PI3K) family, contains phosphorylation sites that regulate the activity of this kinase [7].
Rapamycin and its analogs (called rapalogs) act as allosteric inhibitors of mTORC1 by interacting with the FRB domain of mTOR via FKBP12 protein (FK506-binding protein 1 A 12 kDa) [6]. Furthermore, mTORC2 is insensitive to rapamycin inhibition, as initially described [8], but prolonged exposure to this macrolide results in the disruption of the assembly and integrity of mTORC2, thereby causing the functional inhibition of the complex [9].
Additionally, mTORC1 and mTORC2 share several common proteins, including the catalytic subunit mTOR, Deptor (DEP-domain-containing mTOR interacting protein), mLST8 (mammalian lethal with Sec13 protein 8), and Tti1/Tel2 complex [10] (Figure 1). In addition, each complex has specific proteins. Raptor (regulatory-associated protein of mTOR) and PRAS40 (proline-rich Akt substrate 40 kDa) are specific subunits of mTORC1, while Rictor (rapamycin-insensitive companion of mTOR), mSin1, and Protor1/2 are exclusive to mTORC2 [7] (Figure 1). All of these proteins have different functions in the complexes. Not only do they have structural functions (stabilizing the complexes and recruiting mTOR substrates) but they also contribute to regulating mTOR activity. Recently, an advancement in our understanding of the precise functions of each mTOR companion protein beyond the strictly structural function has occurred. Recent studies demonstrate an important impact in the fine-tuned activity of mTOR kinase, according to post-translational modifications of some of the companion proteins, mainly by phosphorylation.
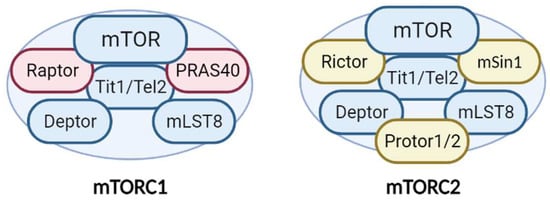
Figure 1. Structure and components of the mTORC1 and mTORC2 complexes. The mTORCs share common proteins (blue) called mTOR (catalytic subunit), Deptor (mTOR inhibitor subunit), mLST8 (scaffold and activator subunit), and Tit/Tel2 (assembly subunit). The specific mTORC1 proteins (pink) include Raptor and PRAS40 (mTOR inhibitor). Rictor, mSIN1, and Protor1/2 (activity modulator) comprise mTORC2 (yellow). See text for more details.
Deptor is an inhibitor of mTOR. The ablation of Deptor increases tumor size and causes the proliferation, migration, and invasion of tumoral cells because of the resulting overactivation of mTOR [11]. Furthermore, several post-translational modifications of Deptor can affect its inhibitory function. Recent studies show that the phosphorylation of Deptor at Tyr289 increases mTOR activity by preventing the correct coupling of the kinase to its protein-binding partner to form a complex [12][13][12,13]. This observation reveals the involvement of a novel molecular switch in the fine regulation of mTORCs.
The importance of mLST8 in mTORC activity is under debate. Studies using Drosophila melanogaster reveal that mLST8 is essential for mTORC2 activity, since LST8 knockout conserves mTORC1 but not mTORC2 activity [14]. It has been proposed that mLST8 specifically affects the interaction between mTOR and Rictor and is essential for the proper assembly of mTORC2 [15]. However, a recent study supports the notion that mLST8 is equally essential for ensuring the stability and activation of the two complexes through a mechanism that involves the kinase Akt [16]. PRAS40 is a negative regulator of mTORC1. Akt phosphorylation of PRAS40 increases the inhibitory effect on mTORC1, measured as an increase in autophagy [17]. Furthermore, mSin1 has been considered a scaffold protein with no relevance for mTOR activity [18]. However, recent data suggest that mSin1 is more than a structural protein. Indeed, it is essential for Akt phosphorylation at Ser473 by mTORC2 [19]. In addition, mSin1 defines the subcellular location of mTORC2, which also determines the final activity of the kinase. Alternative splicing of mSin1 generates five isoforms, of which at least three are able to assemble into mTORC2 and which determine three distinct mTORC2 complexes. These can be located in different subcellular compartments and their sensitivity to activation by PI3K differs [19].
From a functional perspective, mTORCsare considered molecular sensors of cellular energy status. They monitor several extra- and intra-cellular factors to orchestrate a response to maintain cellular homeostasis [20]. Furthermore, mTORC1 is involved in key cellular anabolic and catabolic functions, such as the synthesis of macromolecules (both lipids and proteins), cellular growth, autophagy, and cell cycle progression (Figure 2). In contrast, mTORC2 is related mainly to cell survival, as well as cytoskeletal organization and remodeling [21] (Figure 2). Therefore, both mTORCs are essential for cell viability, as evidenced using knockout mice for mTOR, Raptor, or Rictor. In all of these mouse models, embryo viability is severely compromised [22][23][24][25][22,23,24,25].
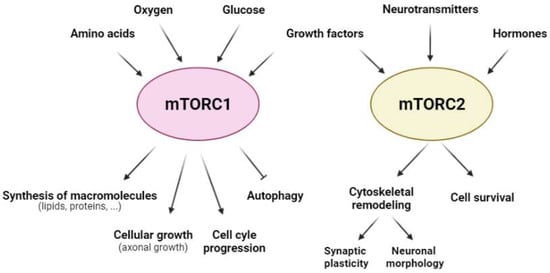
Figure 2. Regulatory factors of mTORC1 and mTORC2 and the cellular responses that they govern. The activity of mTORC1 (pink) and mTORC2 (yellow) is modulated by a range of extra- and intra-cellular factors; mTORC1 (pink) is regulated mainly by amino acids, oxygen, glucose, and growth factors, whereas mTORC2 (yellow) is dependent on growth factors, neurotransmitters, and hormones in the CNS. Furthermore, mTORC1 regulates anabolic and catabolic cellular processes, such as the synthesis of macromolecules (lipids and proteins), cellular growth, cell cycle progression, and autophagy; mTORC2 modulates cellular processes that involve cytoskeletal remodeling, such as synaptic plasticity and neuronal morphology, as well as cell survival.
As both mTORC1 and mTORC2 regulate important cellular functions, their activity is finely regulated. Knowledge of the regulation of mTORC1 is currently much greater than that of mTORC2. Given that the latter is an important player in the maintenance of cell survival and may be an interesting target in neurodegenerative diseases, itwe consider that greater efforts should be devoted to unraveling the fine details of mTORC2 regulation.
The principal mechanism of mTOR activation are related to post-translational modifications (mainly phosphorylation). The mTORCs have multiple regulatory phosphorylation sites, not only in the catalytic subunit (mTOR), but also in other subunits of the complex, such as Raptor, Rictor, Deptor, and PRAS40 [26][27][28][26,27,28]. The data available point to a relationship between the degree of phosphorylation of each subunit and the activity levels of mTOR. This notion opens up a new perspective of the fine regulation of mTOR activity, which could be modulated similarly to volume control and would differentially affect the phosphorylation levels of the substrates and their activities. It is important to keep this perspective in mind in the context of brain ischemia, since the energy status of cells changes rapidly and heterogeneously depending on the degree of injury [29].
2. Upstream Regulatory Pathways of mTORCs
One of the most remarkable characteristics of mTORCs, especially mTORC1, is that these complexes are cellular energy sensors, and as such they act as signal convergence centers from extra- and intra-cellular “energetic factors”. The presence or absence of these factors modulates the final activity of mTORC1, allowing it to trigger distinct cellular responses to modify the balance between anabolism and catabolism, adjusting it to cellular needs (Figure 3).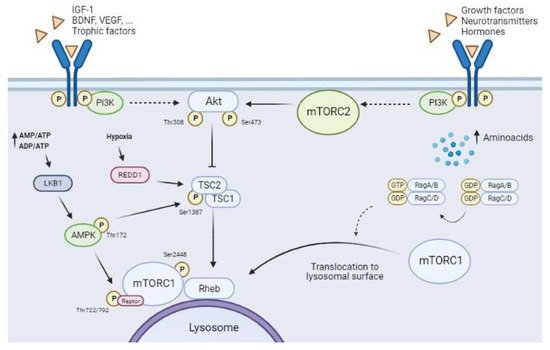
Figure 3. Upstream mTORCs pathways. Solid arrows show direct interactions between proteins and dashed arrows indicate the presence of other unrepresented mediators. The canonical PI3K/Akt pathway regulates mTORC1 activity through the binding of trophic and growth factors (BDNF, VEGF, IGF-1 among others) to RTK/GPCR-specific receptors. PI3K activation upregulates Akt by phosphorylation of Thr308. Full Akt activity requires the phosphorylation of Ser473 by mTORC2. Active Akt inhibits the tuberous sclerosis complex (TSC), which leads to induction of the GTPase Rheb, allowing activation of mTORC1. Amino acid availability leads to the translocation of mTORC1 to the lysosome membrane, which in turn allows its activation by Rheb. The AMPK pathway is activated in low-energetic states (AMP/ATP and ADP/ATP ratios increase). This activation requires the phosphorylation of Thr172 by LKB1. Active AMPK reduces mTORC1 activity through two distinct mechanisms, namely phosphorylation of Raptor at Ser722/792 and phosphorylation of TSC2 at Ser1387. Hypoxia induces an increase in REDD1, which downregulates mTORC1 by destabilizing TSC. Furthermore, mTORC2 activity is regulated by the presence of growth factors, neurotransmitters, and hormones, which all activate PI3K by binding to RTK/GPCRs.
2.1. The Canonical Pathway: PI3K/Akt/mTORC1 and Growth Factors
This pathway senses the availability of several growth or survival factors, including nerve growth factor (NGF), brain-derived growth factor (BDNF), vascular endothelial growth factor (VEGF), insulin, insulin-like growth factor-1 (IGF-1), and neurotrophins (NT-1, -3, and -4). These molecules activate the PI3K/protein kinase B (PI3K/Akt) pathway by binding to their specific membrane receptors, which belong to the tyrosine kinase receptor superfamily (RTK), or to G-protein-coupled receptors (GPCRs) [20]. Full Akt activation requires its phosphorylation at two sites, namely Thr308 via the PI3K/Akt pathway and Ser473 via mTORC2 activity (Figure 3). First, Akt must be recruited to the cellular plasma membrane by direct interaction with phosphatidylinositol (3,4,5)-triphosphate (PIP3) [31][33]. The Ser473 phosphorylation site reveals the exquisite relationship between the activities of the two mTORCs, as mTORC1 activation requires full Akt activity, which in turn calls for previous mTORC2 activation [32][34]. Some studies show that Akt activity is finely regulated and that this protein kinase requires phosphorylation at other residues, such as Ser477 and Thr479, to enhance its interaction with mTORC2 and stability [31][33].
Akt activation induces the inhibition of TSC (Figure 3), a trimeric complex formed by TSC1 (hamartin), TSC2 (tuberin), and the scaffold protein TSC1D7. Several pathways converge on TSC to regulate (both positively and negatively) mTORC1 activity [27]. TSC is a GTPase-activating protein for the small GTPase Rheb (Ras homolog enriched in brain). Rheb is present in an inactive or activated state by binding to GDP or GTP, respectively [33][35]. Akt phosphorylates TSC2 at Thr1462, thereby disassembling and inactivating the complex. This process allows a Rheb-GTP-active state, which consequently induces mTORC1 through an unknown mechanism (Figure 3) [33][34][35,36]. The subcellular localization of mTORC1 in this step is essential for its full activation, as previously mentioned (see Section 1.1), since Rheb-GTP is located in the lysosomal membrane (see Section 1.2.3) (Figure 3).
2.2. AMPK–mTORC1 Pathway: The Glucose Sensor
AMPK, a negative regulator of mTORC1 activity, comprises three subunits (α, β, and γ), and it senses cellular energy status and glucose availability [35][37]. The highly energy-demanding brain is unable to store glucose and is greatly dependent on constant glucose supply from blood. A decrease in glucose supply induces a reduction in the AMP/ATP and ADP/ATP ratios, which is sensed by AMPK (Figure 3). This detection induces AMPK activation by phosphorylation at Thr172 of its α-subunit by LKB1 tumor suppression kinase [36][38]. Ca2+-activated Ca2+/calmodulin-dependent kinase β (CaMKKβ) and transforming growth factor-β-activating kinase 1 (TAK1) are both activators of AMPK [37][38][39,40].
The activation of AMPK inhibits mTORC1 activity through phosphorylation on two targets, namely TSC2 and Raptor [39][41]. Therefore, AMPK regulates mTORC1 activity at two levels (Figure 3). On the one hand, AMPK phosphorylation of TSC2 at Thr1227 and Ser1345 improves the stability of the TSC complex [33][35], promoting the inactive state of Rheb-GDP and inducing the inhibition of mTORC1 activity [40][42]. On the other hand, Raptor phosphorylation at Ser722/792 by AMPK disrupts mTORC1 and induces its inhibition [41][43]. AMPK activation and mTORC1 inhibition lead to autophagy (see Section 1.3).
A reduction in oxygen levels, as occurs under hypoxia, decreases cellular ATP levels by inhibiting oxidative phosphorylation and other metabolic programs. This scenario promotes an ATP/AMP imbalance, inducing AMPK activation [42][44] and mTORC1 inhibition.
2.3. REDD1 and mTORC1
Reduced oxygen availability is another scenario that negatively modulates mTORC1 [43][45] (Figure 3). Hypoxia induces an increase in regulated DNA damage and development 1 (REDD1; also known as RTP801/DDIT4) expression, a highly conserved stress-response protein [44][46]. REDD1 downregulates mTORC1 through a TSC-dependent mechanism [45][46][47,48]. REDD1 triggers the release of TSC2 with the adapter protein 14-3-3, stabilizing the interaction between TSC1 and TSC2 and inducing mTORC1 inhibition [45][47]. Increased REDD1 expression early after ischemia has been described in neurons and glial cells [47][49].
2.4. Regulation of mTORC1 by Amino Acid Levels
Amino acids are key elements for neural cells. Moreover, amino acids are another regulatory pathway of mTORC1 involving a molecular mechanism that is not fully understood. Amino acids levels have an important influence on mTORC1 activity as they mediate the translocation of these kinases to the lysosomal membrane, a necessary step for it activation [21][34][21,36]. Rag GTPases have been reported to be involved in this process [21][34][48][49][21,36,50,51]. These molecules are small G-proteins that belong to the Ras superfamily andare present as heterodimers—RagA/B dimerized with RagC/D. The active conformation of these Rag heterodimers is RagA/B binding to GTP (RAG-A/BGTP) and RagC/D binding to GDP (RAG-C/DGDP) (Figure 3) [50][52]. Amino acid availability allows the active conformation of Rag, which binds directly to Raptor and induces the recruitment of mTORC1 to the lysosomal membrane [51][53] (Figure 3). At this location, mTORC1 is accessible to Rheb as a result of their proximity, and consequently mTORC1 is activated [52][51][30,53]. Leucine is a key amino acid that influences mTORC1 activity as it enhances the stabilization of the Raptor–mTOR interaction [53][54].
Cerebral ischemia is followed by a reduction in blood flow to the brain. This disruption causes the dysregulation of all upstream pathways that regulate mTORC1 (see Section 2). However, the impact of each individual upstream pathway on the activity of this complex is unknown. In this regard, it would be interesting to determine the relevance of each pathway for the final activity of mTORC1, which could differ between cell types. Preliminary data obtained in our laboratory using primary cultures of neurons and astrocytes suggest that the reductions in several energetic factors (glucose, oxygen, and trophic factors) have a summative effect on the activity levels of mTORC1.
3. Downstream Targets of mTORCs
The strategic position of mTORC1, downstream of three important signaling pathways, makes this complex an essential convergence center to check cell energy status. HIn this serction, we describe the signaling pathways downstream of mTORC1, their principal targets, and the main cellular processes that they regulate. In addition, herwe provide the scarce details available for the downstream pathways of mTORC2 in the CNS.
One of the best known cellular processes regulated by mTORC1 is protein synthesis (Figure 4), which is essential for neural cell survival, synaptic plasticity, and brain development, and is dysregulated in several brain conditions, such as ischemia [27]. In neurons, trophic factors such as BDNF, insulin, and IGF-1, as well as some neurotransmitters, induce an increase in protein synthesis by local mTORC1 activation [54][55][55,56]. Protein synthesis is regulated by two well-characterized targets of mTORC1, called p70 ribosomal protein S6 kinase (P70S6K) and eukaryotic initiation factor 4E (eIF4E)-binding proteins (4EBPs), which trigger elongation and initiation of protein translation, respectively [56][57] (Figure 4). Furthermore, mTORC1 phosphorylates 4EBPs at several residues, thereby allowing the release of a group of eukaryotic initiation factors (eIFs) located on 5’-UTR of cap-dependent mRNA that initiates translation [57][58][58,59]. Three isoforms of 4EBP have been described, namely 4EBP-1, 4EBP-2, and 4EBP-3, with 4EBP-2 being the most common in the CNS [58][59]. Additionally, mTORC1 phosphorylates 4EBPs mainly at Thr37/46 and Ser65. It has been proposed that hierarchical phosphorylation of 4EBPs may be crucial in the fine regulation of translational processes mediated by mTORC1 [59][60][61][60,61,62]. P70S6K is the other main substrate of mTORC1 related to protein synthesis. Additionally, mTORC1 phosphorylates P70S6K at Thr389, thereby allowing the recruitment of the 40S ribosomal subunit to the translational machinery. Furthermore, P70S6K phosphorylates eukaryotic initiation Factor 2 kinase (eIF2K), which induces the elongation phase of protein synthesis [21].
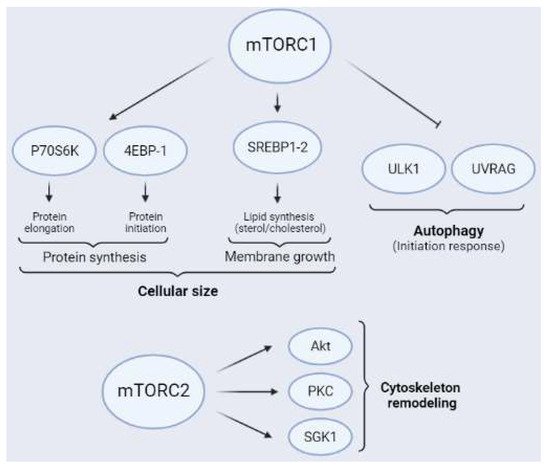

Figure 4. Schematic representation of the main mTORC1 (above) and mTORC2 (below) substrates. The main targets of mTORC1 related to protein synthesis are P70S6K and 4EBPs. Furthermore, mTORC1 regulates lipid synthesis through the transcriptional factor of lipogenesis SREBP1-2 and inhibits autophagy through ULK1 and UVRAG, the latter mainly in neurons. Additionally, mTORC2 is an activity modulator of Akt, PKC, and SGK1, all of which are related to cytoskeleton remodeling.
The synthesis of structural lipids allows the length of the plasmatic membrane to increase, a key aspect in axonal growth, dendritic arborization, and myelination (Figure 4). In addition, lipids are important molecules in cellular metabolic processes, since they are sources of energy, especially when glucose is lacking, as occurs after ischemia [62][63][64][63,64,65]. Furthermore, mTORC1 inhibition using rapamycin downregulates lipid synthesis. This observation, reveals that mTORC1 participates in this process [65][66]. Additionally, mTORC1 activates sterol regulatory element-binding proteins 1 and 2 (SREBP1-2) through P70S6K, since ablation of P70S6K induces reductions in the amount of lipid synthesis and cell size [66][67]. Activated SREBP is translocated to the nucleus to act as a transcription factor of genes involved in lipogenesis [67][68][68,69]. In the developing brain, mTORC1 induces the transcription of numerous genes related to the sterol–cholesterol biosynthesis pathway. Altered expression of these genes by dysregulation of mTORC1 contributes to neurodevelopmental disorders [69][70] and also affects myelination [70][71], an essential process for neuronal recovery after cerebral ischemia (see Section 2.2.3).
Autophagy is a highly conserved cellular catabolic mechanism that involves the degradation of damaged cellular components, misfolded proteins, long-lived proteins, and damaged organelles by lysosomes. It is believed that after injury, autophagy plays a critical role in removing damaged molecules and subcellular components to maintain cellular homeostasis [71][72]. Furthermore, mTORC1 is a key player in the regulation of autophagy (Figure 4). It induces this catabolic mechanism and facilitates the fusion of the autophagosome with the lysosome, a key step in this process [72][73]. In nutrient-rich conditions, active mTORC1 inhibits autophagy by phosphorylating Unc-51-like kinase 1 (ULK1) or UV radiation resistance-associated gene protein (UVRAG), among others [73][74][75][76][77][74,75,76,77,78]. Inversely, in nutrient-poor conditions, reduced mTORC1 activity induces autophagy, which leads to the removal of proteins and organelles to compensate for nutrient starvation. As itwe described previously, a reduction in glucose levels induces AMPK activation, which phosphorylates Raptor, which in turn inhibits mTORC1 and triggers autophagy [72][78][79][73,79,80]. Inactivation of mTORC1 under starvation conditions prevents the maintenance of Ser757 phosphorylation of ULK1, which induces autophagy initiation [80][81]. As a general idea, autophagy induction after ischemia could be considered an intrinsic mechanism of neuroprotection. In this regard, some experimental evidence confirms this hypothesis [78][79]. The pharmacological induction of autophagy after ischemia using rapamycin reduces brain damage [72][81][82][83][73,82,83,84]. However, other results indicate that excessive autophagic flow aggravates ischemic damage [84][85]. Therefore, autophagy is another example of a cellular mechanism that requires fine regulation to have positive or negative effects after injury.
The mTOR activity plays a pivotal role in axonal growth, a highly regulated process involved in synaptic plasticity and development [85][86]. Rapamycin administration to primary cultures of neurons induces the inhibition of axonal growth and prevents neuronal differentiation [30][32]. However, mTORC1 activation by ablation of their negative upstream regulators (TSC or PTEN) stimulates regenerative processes related with axon guidance and growth [86][87][87,88]. In this regard, the presence of some components of the mTORC1 pathway, including P70S6K and 4EBP-1, has been reported in the axonal cones of primary neurons. This interesting observation reflects the importance of mTORC1 location in the appropriate subcellular compartment to regulate certain neuron-specific mechanisms, such as axonal growth, in situ [26].
The downstream targets of mTORC2 include several members of the AGC kinase family, such as Akt, PKC, and SGK1 [88][89] (Figure 4). As mentioned previously, mTORC2 participates in neuronal cytoskeleton remodeling, specifically in the actin cytoskeleton [8]. Rictor ablation induces a reduction in mTORC2 activity, thereby having impacts on neuronal size and morphology [89][90][90,91]. Indeed, cytoskeleton rearrangement is a crucial factor for synaptic plasticity [91][92].
