This enartry mainly describesicle is a review of the patent ductus arteriosus(PDA) in preterm infants with a special focus on it's effect on pulmonary vasculature. It also reviews the role of cardiac catheterization as a diagnostic tool to evaluate the hemodynamic effects of a longstanding large PDA. It introduces the possible management strategies available in the cardiac catheterization laboratory for a preterm infants with a PDA and pulmonary hypertension.
- PDA, Pulmonary hypertension, Preterm Infant
- Extremely Low Birth Weight Infant
Note:All the information in this draft can be edited by authors. And the entry will be online only after authors edit and submit it.
Definition: There continues to be a reluctance to close the patent ductus arteriosus (PDA) in premature infants. The debate on whether the short-term outcomes translate to a difference in long-term benefits remains. This entry intends to review the pulmonary vasculature changes that can occur with a chronic hemodynamically significant PDA in a preterm infant. It also explains the rationale and decision-making involved in a diagnostic cardiac catheterization and transcatheter PDA closure in these preterm infants.
1. Introduction
In extremely low birth weight (ELBW) infants, the incidence of pulmonary arterial hypertension (PAH) after a month of age ranges 4% to 16% [1]. The presence of severe PAH with bronchopulmonary dysplasia (BPD) in a preterm infant bestows a 40% mortality by 2 years of age [2]. It is difficult to predict the risk of developing PAH in preterm infants as it is multifactorial, with conditions such as BPD, necrotizing enterocolitis (NEC), and sepsis being risk factors [3]. In addition to these factors, there is an enhanced risk of developing PAH early in premature infants with post-tricuspid shunts [4]. The common post-tricuspid shunts include ventricular septal defects (VSD), atrio-ventricular septal defects and patent ductus arteriosus (PDA). The two important factors for outcomes after closure of these defects are the age at the time of closure and the presence of pre-operative pulmonary vascular disease (PVD) [5]. Although there is a general notion that irreversible PAH does not develop in the first year of life, the progression of PAH can be unpredictable in ELBW infants with multiple co-morbidities. The effects on the pulmonary vasculature from increased pulmonary blood flow in a long-standing, large, hemodynamically significant PDA (hsPDA) may be more detrimental than a VSD due to the high-pressure, pulsatile flow throughout the cardiac cycle in a PDA [6]. Management of PAH in preterm infant with a large hsPDA is challenging. This review attempts to highlight the pulmonary vascular changes that can be associated with an hsPDA in a preterm infant. It also introduces diagnostic cardiac catheterization as a tool to assist in decision-making for PDA closure in select infants.
2. Management of a Large PDA with Pulmonary Hypertension
It is clear that the PDA should not be closed when there is right-to-left shunting secondary to supra-systemic PA pressures. The PDA is in fact beneficial as a pop-off in providing cardiac output at the cost of arterial desaturation. It acts as an outlet to the right ventricle to pump into systemic circulation. Usually, the window of opportunity to close the PDA has been lost at this stage. To help decrease the PVR, pulmonary vasodilator therapy may be used in these patients with the intent to possibly reverse remodel the vasculature. If the PVR shows reactivity and decreases, it may potentially allow for PDA closure in the future.
Infants with an elevated PVR and an hsPDA are difficult to treat. The use of supplemental oxygen to treat the PAH may have conflicting effects in the presence of a large hsPDA. The pulmonary vasodilatory effect of oxygen may potentially increase left-to-right shunting and PBF. This may lead to vascular shear stress and damage. The use of pulmonary vasodilator therapy to boost reversal of vascular remodeling may worsen the shunting. Contrarily, closing a PDA with severe PAH runs the risk of a pulmonary hypertensive crisis or right ventricular failure. In such circumstances, further evaluation is essential to assess whether there is reactivity, and/or reversibility of the PVR. A reactive pulmonary vascular bed may indicate potential regression of PAH after PDA closure. Earlier stages of muscular hypertrophy are reversible, but later stages of intimal proliferation and fibrosis are not. Closing hsPDAs by medical therapy or surgical ligation may be suboptimal as these therapies do not lend proper understanding of the PVR, reactivity to vasodilators, and alterations in hemodynamics with PDA closure.
Role of Cardiac Catheterization
Information from clinical examination, chest X-ray, arterial oxygen saturation, blood gases, and echocardiograms is usually helpful in quantifying the hemodynamic significance of a PDA and mild PAH. Moderate-to-severe PAH is often arduous to quantify accurately by the aforementioned tests to conclusively validate the safety of PDA closure regardless of the method of closure. There are strategies using echocardiography to evaluate the PDA flow in the presence of PAH and a large PDA. If bidirectional shunting goes to all left to right shunting with 100% supplemental O2 or iNO (inspired nitric oxide), it suggests reversibility. In some infants, the non-invasive clinical data are equivocal. This is where cardiac catheterization can be of value. It offers the opportunity for meticulous direct hemodynamic assessment including evaluation of vasoreactivity and reversibility of the pulmonary vasculature to vasodilators. Based on these data, the clinician can assess whether a PDA needs to be closed and whether there will be benefit to PDA closure in the presence of PAH. It also provides a therapeutic option for trans-catheter PDA closure (TCPC) in ELBW infants [7][8][9][10][11][14,15,16,17,18]. It has the capability to test the real-time hemodynamics after temporarily occluding the PDA, i.e., “test occlusion” thus enabling appraisal of the safety of PDA closure in infants with PAH. This is not possible with medical therapy or surgical ligation. It can also evaluate or confirm pulmonary vein stenosis, which is known to develop in a small percentage of preterm infants with BPD, as this can be challenging to affirm through echocardiography at times. This decision to go ahead with cardiac catheterization has to be weighed in with the risks of transport to the cardiac catheterization laboratory and the risks of the procedure [7][14].
The Pediatric Pulmonary Hypertension Network (PHN) has outlined a protocol for diagnostic cardiac catheterization for infants with BPD and pulmonary hypertension (PH) [12][19]. Under baseline conditions, saturation and pressure data are obtained. A Qp:Qs (pulmonary-to-systemic blood flow ratio) is calculated to assess the degree of shunting. Systemic vascular resistance (SVR) and PVR are calculated and indexed (PVRi). A PVRi > 3 Wood Unit × m2 is abnormal. A PVR/SVR ratio of >0.5 indicates a high risk for progressive right heart failure or decompensation. To test pulmonary reactivity, these measurements are then repeated with 100% oxygen, 100% oxygen + inspired nitric oxide (iNO) up to 40 ppm (parts per million). Though direct measurements are obtained during a diagnostic cardiac catheterization, there are known limitations in the assessment of cardiac output and PVR. The branch PAs have different saturations, as there is differential PBF from the PDA. Oxygen consumption is estimated in premature infants and, hence, may not be accurate. This exercise of hemodynamic assessment and vasoreactive testing is only done if there is suspicion of high PVR from pre-procedure assessment as it adds to the procedure time.
Test occlusion of the PDA to assess safety for TCPC can be done by two methods. It can either be done by balloon occlusion or by using the anticipated device itself (Figure 1). Balloon occlusion, if not well positioned and stable, has the disadvantage of inadvertently causing aortic or pulmonary obstruction and may make the assessment of hemodynamic data challenging. We prefer device test occlusion. The appropriately sized device is chosen based on the size of the PDA by echocardiography and pulmonary angiography. This procedure is reserved only for cases where the hemodynamics are borderline and the interventionalist wants to confirm that closing the PDA will be well tolerated hemodynamically by the infant. The three important parameters after test occlusion of the PDA to assess safety are as follows:
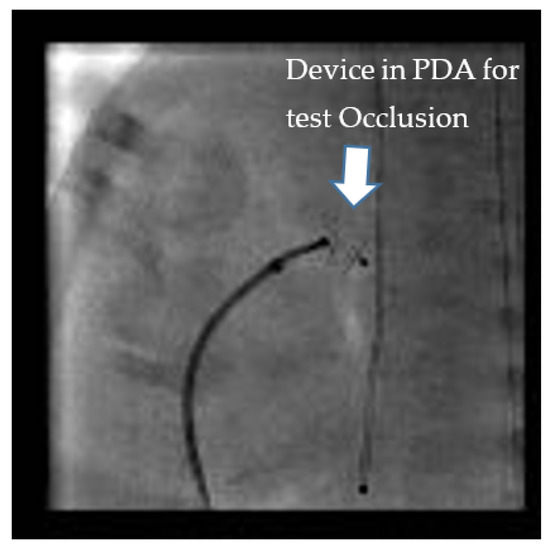
Figure 1. “Test Occlusion”: Testing the hemodynamic effects of temporary occlusion of the patent ductus arteriosus (PDA) during cardiac catheterization. The ideal response is a drop in pulmonary artery pressure by 20% with no drop or an improvement in systemic blood pressure.
(a)20% drop in the baseline systolic PAP or at least no increase in systolic PAP.
(b)No drop in the systemic pressure. This suggests that the PDA was not required for providing cardiac output.
(c)No drop in oxygen saturation or clinical signs of a PH crisis [20,21].
-
If the hemodynamics are favorable, TCPC allows for the aggressive use of pulmonary vasodilator therapy to help with vascular remodeling. The vasodilator therapy also helps to preserve right ventricular function by decreasing right ventricular afterload. Unfortunately, a small percentage of preterm infants who usually have a PVR/SVR ratio >0.5 will have a less than 20% drop in PAP and a small decrease in cardiac output with test occlusion. These infants are more likely to clinically decompensate after TCPC as their PAH does not regress, and they are at risk for progressive right ventricular failure [13][22]. Therefore, there should be a low threshold to refrain from closing the PDA if the hemodynamic response to test occlusion is borderline, i.e., the PAP falls less than 20% or the systolic blood pressure falls, suggesting that the PDA was needed to augment cardiac output. Selecting infants with reversible PAH is important, since they are the ones who more often benefit from PDA closure. For borderline cases (higher PVR), the use of vasodilator therapy for a couple of weeks prior to PDA closure is thought to have favorable outcomes [8][15]. In select infants with a high baseline PVR and bidirectional PDA shunting, if 100% oxygen and iNO vasodilate the pulmonary vasculature adequately, leading to pure, left-to-right shunting, increased Qp:Qs ratio, and a drop in PVR, PDA closure may be beneficial to help introduce pulmonary vasodilator therapy. However, the baseline bidirectional shunting may be concerning for potential right ventricular failure with complete closure of the PDA. In these circumstances, based on our previous animal experiment [14][23], we have preferred to fenestrate the device by cutting out the Gore-Tex membrane off two cells of the device as shown in Figure 2A,B. This provides a pop-off and may prevent right ventricular failure whilst providing partial PDA closure in order for pulmonary vasodilators to be used for vascular remodeling.
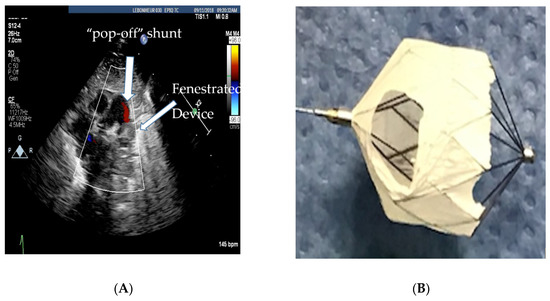
Figure 2. (A) Echocardiogram showing the fenestrated MVP-5Q device with the residual “pop-off” shunt, (B) fenestrated microvascular plug (MVP-5Q).
Our institutional experience [15][16] [24,25] with TCPC and hemodynamic testing in ELBW infants (<1000 g at birth, and less than 27-weeks of gestational age) with an hsPDA has showed a difference in hemodynamics based on the time of the procedure from birth. Those who had TCPC at less than 4 weeks of age had a higher Qp:Qs (median 2.5:1) suggestive of a significant shunt, and a lower PVR (1.6 WU·m2) in comparison to that of those that had TCPC after 8-weeks of age (median Qp:Qs of 1.8:1, and median PVRi of 3.3 WU·m2). Infants with PAH were referred later for TCPC (median procedure age of 84 vs. 32 days). Infants with PAH, though older than those without PAH at the time of TCPC, took longer to extubate after PDA closure. In addition, earlier closure, as expected, allowed for better growth velocity. The mean weight gain between 4 and 8 weeks of age was 25 g/day for the early closure group in comparison to 16 g/day for the group who had TCPC after 8 weeks of age [16][25]. There is also a misconception that it is technically easier to close the PDA via the transcatheter route in an older infant, which may often be the reason for delayed referral for TCPC. In our experience, pre-procedural risk factors for PDA closure are relatively worse baseline respiratory status and PAH, both of which are seen in ELBW infants referred for TCPC at a later age. There may be benefit in closing hsPDAs earlier, before the development of elevated PVR in ELBW infants. Although our experience continues to be limited by long-term outcomes and controlled trials, there are vital short-term benefits worth considering as mentioned above. One must also be mindful that PAH in preterm infants is multifactorial and that an hsPDA is a contributing factor.
3. Conclusions
Persistence of a large hsPDA in ELBW infants could accelerate the development of PAH. Pulmonary vascular changes, even when reversible, may take time to normalize. Early closure within the first 4 weeks of life preceding the development of significantly elevated PVR, may be considered helpful. In select preterm infants with at-least moderate PAH, cardiac catheterization is a good armament to the clinician when the data are equivocal and provides a therapeutic option for patient selection for safe ductal closure. By eliminating the large shunt, aggressive pulmonary vasodilator therapy for PAH can be initiated. A small subset of patients with severe PAH and borderline hemodynamics may not benefit from PDA closure and may eventually develop right heart failure from progressive pulmonary vascular disease. Identifying this small percentage of patients by meticulous vasodilator testing, test occlusion, and partial closure of the PDA is a promising new strategy that is only possible during cardiac catheterization.
