SARS-CoV-2 is a member of the Coronaviridae family, a group of enveloped, positive-sense, single-stranded RNA viruses. Many extrapulmonary manifestations of the coronavirus disease 2019 (COVID-19) have been reported involving the cardiovascular, renal, gastrointestinal and urinary systems. These widespread manifestations are attributed to the presence of the ACE2 (Angiotensin converting enzyme 2) receptor in these tissues, which is postulated to be at the center of the pathogenesis of COVID-19. Similarly, expression of the ACE2 receptor has also been reported in various endocrine tissues including the hypothalamus, pituitary, thyroid, gonads, and pancreatic islets. Therefore, it is imperative to understand the way COVID-19 can alter the function of these tissues and cause pathology, especially considering the close interplay between various endocrine systems as part of the RAAS (renin–angiotensin–aldosterone system) pathway and the central role of ACE2 in this pathway.
- COVID-19
- endocrine
- misinformation
- pregnancy
- infertility
1. COVID-19 and Endocrine System
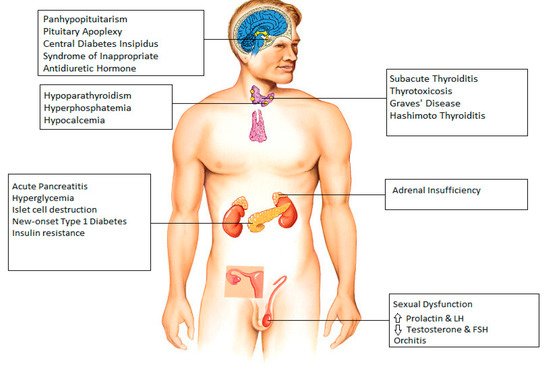
2. Hypothalamus and Pituitary
3. Thyroid
4. Parathyroid
5. Pancreas
6. Adrenal Gland
7. Gonads
8. Endocrinopathies and COVID-19 Vaccines
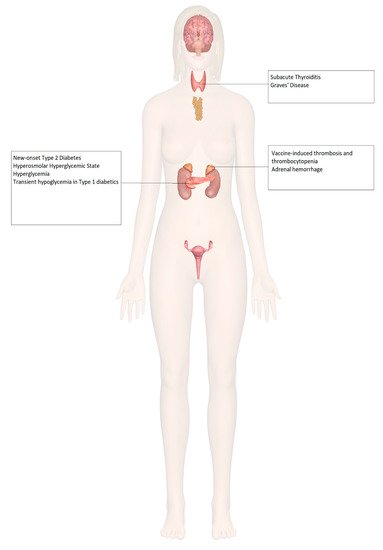
9. Adrenal Insufficiency
10. Diabetes Mellitus
11. Thyroid Disorders
12. Hypogonadism and Infertility
13. Osteoporosis
14. Misinformation: COVID-19 Vaccine and Endocrine System
| Author | Study Design | Criteria | Patient Population | Conclusion | Diagnosis | Complications | Treatment | Outcome | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Limitations | ||||||||||||||||
| Taylor et al. (Wales, 2021) [69][71] | Adrenal | 38, Male | Astra Zeneca | 8 days after Dose 1 | Severe abdominal pain Vomiting |
Vaccine–Induced Thrombosis and Thrombocytopenia with Bilateral Adrenal Hemorrhage | Dural venous sinus thrombosis | Intravenous Immunoglobulin, Hydrocortisone, Argatroban Plasma exchange |
Improved platelet count after plasma exchange | |||||||
| Gonzalez et al. [86][88]. | Single-center prospective study | Inclusion: Men aged 18–50, no underlying fertility issues. |
45 male participants. | No significant decrease in sperm parameters after 2 doses of COVID-19 vaccination. |
|
|||||||||||
| Boyle et al. (United Kingdom, 2021) [70][72] | Adrenal | 55, Female | Astra Zeneca | 8 days after Dose 1 | Left iliac fossa pain Vomiting |
Left Adrenal Hemorrhage | Thrombo-embolism in both lungs, left basilic vein, and left renal vein | Hydrocortisone Apixaban |
Exclusion: Positive response to therapy, conservatively managed further |
|||||||
| COVID-19 symptoms or positive results within the last 90 days. | Abu-Rumaileh et al. (Jordan, 2021) [76][78] | Diabetes | 58, Male | |||||||||||||
| Lifshitz et al. [87][89]. | Pfizer/BioNTech | 21 days after Dose 1 (2 days after Dose 2) | Nocturia | Prospective cohort study | Inclusion: Men < 45 years old, fertile men were considered to be those who had previously successfully impregnated their partners without the use of artificial reproductive technology. Polyuria |
75 male participants. | Polydipsia Altered mental status |
Semen parameters found to be within normal parameters after COVID-19 vaccination. | Weight loss |
| Hyperosmolar Hyperglycemic State |
| IV Fluids Insulin drip Glargine 50 units daily plus 10 units pre-meal insulin |
Insulin tapered and stopped in 4 weeks Metformin continued with good glycemic control |
||
| Mishra et al. (India, 2021) [77][79] | Diabetes | 58, Female | ||||||||||||||
| Exclusion: | Covishield | 1 day | Previously diagnosed with SARS-CoV-2 infection, taking medications known to be detrimental to semen parameters. | None Hypertension and tachycardia None |
Exacerbation of hyperglycemia in pre-existing Type 2 Diabetes Mellitus | None | Increased dose of Metformin in patient 1 No interventions in patients 2 and 3 |
Return to previous blood glucose levels in 1 month, 3 days and 15 days, respectively | ||||||||
| 64, Male | ||||||||||||||||
| Lipkind et al. [91][93] | Retrospective cohort study | Inclusion: Single-gestation pregnancies. |
46,079 participants. | COVID-19 vaccination during pregnancy was not significantly associated with increased risk for preterm birth overall or SGA at birth |
|
1 day | ||||||||||
| Exclusion: Age < 16 or >49 years, multiple gestations, no documented care in the health system, implausible gestational age, pregnancy start date outside the prespecified periods. |
65, Male | 6 days | ||||||||||||||
| Blakeway et al. [92][94]. | Retrospective cohort study | Inclusion: Pregnant women with known vaccination status, complete maternal and fetal outcome data. |
1328 Participants. | Similar pregnancy outcomes seen in vaccinated and unvaccinated participants. |
|
Heald et al. (United Kingdom, 2021) [78][80] | Diabetes | 20 patients Median age 53 (range 26–70), 11 Females, 9 Males |
||||||||
| Exclusion: Complicated pregnancies with genetic syndromes, fully vaccinated before getting pregnant. | Pfizer/BioNTech | [ | 6][8] Astra Zeneca [10][12] |
7 days | None | Transient hypoglycemia in Type 1Diabetes Mellitus patients | None | No intervention | Return to previous glucose levels in further 7 days | |||||||
| Irlemi et al. (Turkey, 2021) [79][81] | Thyroid | 35, Female | CoronaVac | 4 days after Dose 2 4 days after Dose 1 7 days after Dose 2 |
Anterior neck pain Fever Palpitations Weight loss Fatigue |
Subacute Thyroiditis (secondary to ASIA syndrome) | Recurrent myalgia and neck pain in patient 2 | Methylprednisolone 16 mg once daily propranolol 25 mg twice daily No intervention in patient 3 |
Complete resolution of symptoms | |||||||
| 34, Female | ||||||||||||||||
| 37, Female | ||||||||||||||||
| Franquemont et al. (USA, 2021) [80][82] | Thyroid | 42, Female | Pfizer/BioNTech | 5 days after Dose 1 | Sore throat Palpitation Tachycardia |
Subacute Thyroiditis | None | Prednisone 40mg daily and Propranolol 20mg as needed | Rapid improvement of symptoms after therapy | |||||||
| Oyibo (United Kingdom, 2021) [81][83] | Thyroid | 55, Female | Astra Zeneca | 21 days after Dose 1 | Neck pain Swelling Headache Sore throat Myalgia Palpitation |
Subacute Thyroiditis | None | Levothyroxine 50 mg daily Propranolol |
Resolution of symptoms after therapy | |||||||
| Sahin et al. (Turkey, 2021) [82][84] | Thyroid | 67, Male | Subacute Thyroiditis | |||||||||||||
| Vera-Lastra et al. (Mexico, 2021) |
