1. Introduction
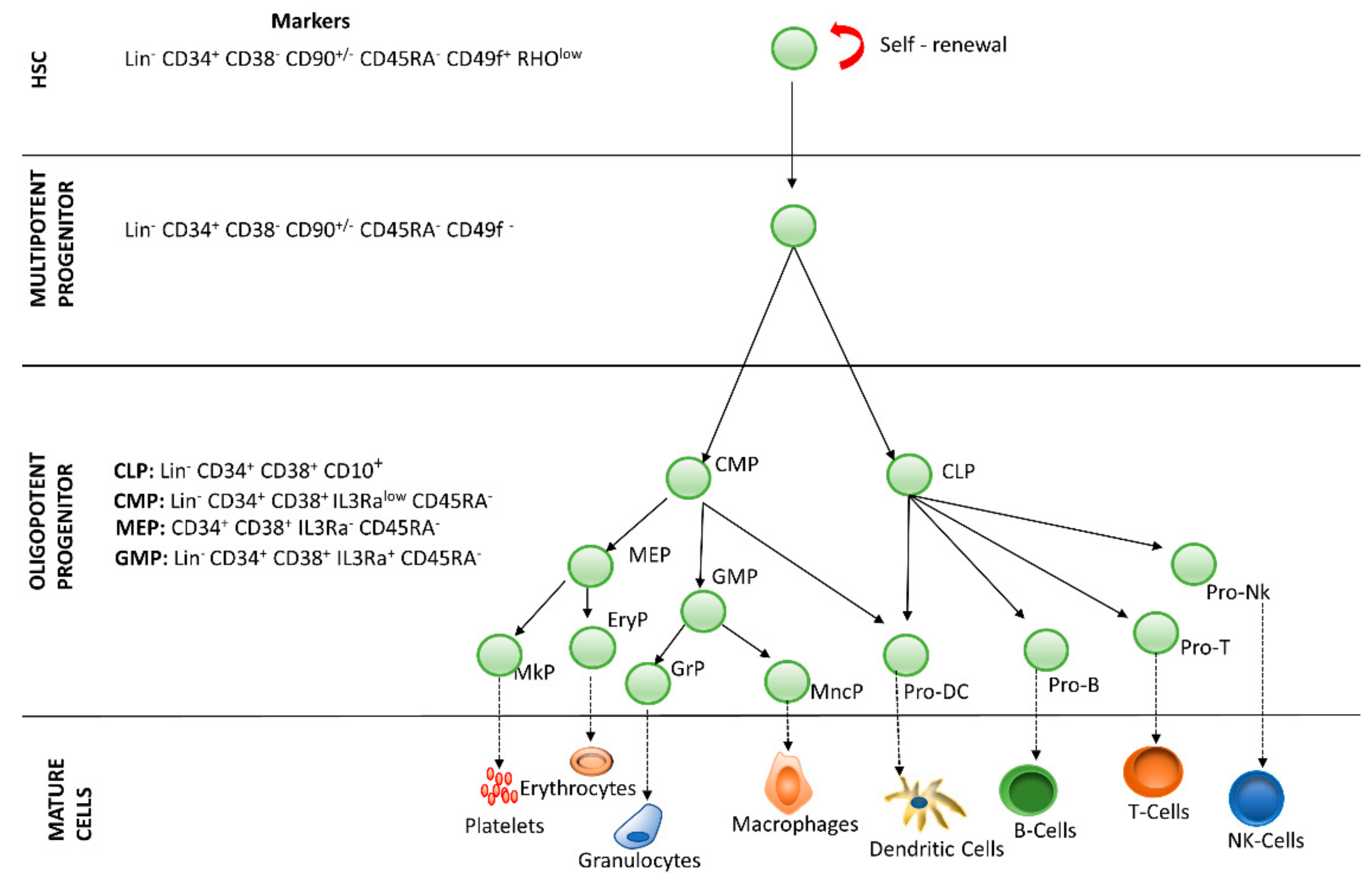
Hematopoiesis is the process by which blood cells are formed, replenishing the blood system over the life of an individual. The hematopoietic process is a highly hierarchical phenomenon, in which hematopoietic stem cell (HSCs) differentiation and proliferation are of vital importance. Each cell within the hematopoietic hierarchy can be distinguished based on specific surface markers, which contain epitopes that are recognized by antibodies [1,2,3]. Figure 1 shows the main markers in human hematopoietic hierarchy. The hematopoietic process in humans starts in the yolk sac (mesoblastic phase). Then, it is transferred to the liver and spleen. Finally, the bone marrow becomes the main organ responsible for hematopoiesis. In the bone marrow, HSCs have the capacity of unlimited self-renewal, producing progeny that is the same as the original cell. They are generally in the G0 phase of the cell cycle and have the capacity to differentiate into specialized cells.
Figure 1. Hierarchy of human hematopoiesis. LT-HSC: Long Term-Hematopoietic Stem Cell; ST-HSC: Short Term-Hematopoietic Stem Cell; MPP: Multipotent Progenitor; OPP: Oligopotent Progenitor; LRP: Lineage Restricted Progenitor; MEC: Mature Effector Cell. The markers of the most important lineages are listed: Common Lymphoid Progenitor (CLP); Common Myeloid Progenitor (CMP); Megakaryocyte-Erythrocyte Progenitor (MEP); Granulocyte-Macrophage Progenitor (GMP). Restricted lineage progenitor cells: Megakaryocyte Progenitor (MkP); Erythrocytic Progenitor (EryP); Granulocytic Progenitor (GrP); Monocyte Progenitor (MncP); Dendritic Progenitor Cell (Pro DC); Progenitor Cell-T (Pro-T); Progenitor Cell-B (Pro-B); Progenitor Cell-Nk (Pro-Nk).
2. Hematopoietic Stem Cells
The medullary microenvironment participates in the quiescence, self-renewal, proliferation, maturation and apoptosis of HSCs and contains several cells (i.e., mesenchymal stem cells, endothelial cells, fibroblasts, osteoblasts, reticular cells, adipocytes). These cells are sources of cytokines, growth factors, glycoproteins and glycosaminoglycans, among other regulators. Different combinations of these molecules lead to the formation of specific microenvironments within the medullary cavity, known as niches [
4]. Histologically defined microenvironments are subdivided into four regions: endosteal, subendosteal, central, and perisinusoidal. Granulocytes and monocytes are found in all regions of the bone marrow, whereas erythroblasts proliferate preferentially in the central region [
5]. Concerning the dynamics of the lymphoid lineage, B lymphocyte precursors are found in the subendosteal region, gradually decreasing toward the central region, whereas mature B cells are found throughout the bone marrow [
6]. HSCs are located in the endosteal region, also known as the osteoblastic niche, but studies suggest that HSCs may migrate to the perisinusoidal region or vascular niche and remain quiescent or differentiate depending on the needs of the organism [
2,
5,
7,
8,
9]. In fact, studies with new markers for HSCs and niche cells, new image techniques, including labeling protocols, have shown that most HSCs reside adjacent to sinusoidal vessels, leading to the proposed existence of a perivascular niche for HSCs [
10]. It is assumed that in the bone marrow, there are at least two different niches: the endosteal niche, which would harbor quiescent HSCs, and the perivascular niche, which would harbor cycling HSCs [
11]. Although most studies have been done on non-humans, researchers suppose that the data reflect what happens in humans. It has previously been proposed that HSCs are maintained in the endosteal (osteoblastic) niche; however, the available evidence does not seem to support this model. Nevertheless, the endosteal niche seems to support the maintenance of a subset of lymphoid progenitors [
10]. Approximately 80% of dividing and non-dividing HSCs have been described to be associated with sinusoidal vessels, with another 10% of HSCs being adjacent to arterioles, and almost another 10% in transition zone vessels [
10]. A small percentage of HSCs are located in the endosteum. In fact, a quantitative model of cellular components that could define these microenvironments, and the preferential location of HSCs in the bone marrow are still lacking [
12]. Obstacles to recognizing the HSCs in the bone marrow include the low frequencies at which HSCs are found in the bone marrow and the cellular complexity of the bone marrow microenvironment.
Cell signaling in the HSC niche is a complex process and passes through an extensive signaling network balancing self-renewal and differentiation [
13]. This signaling involves several substances, such as growth factors and cytokines that are secreted by both the medullary stroma cells and hematopoietic stem cells and are important signaling factors for hematopoiesis, proliferation, and differentiation [
14].
The Jak/STAT, Ras/Erk, and PI3K/Akt signaling pathways have been described as important inducers of erythropoiesis transduction by activation of the erythropoietin receptor, and these intracellular pathways are responsible for the survival, proliferation, and differentiation of normal erythropoietic progenitors [
15].
Other intracellular signaling pathways that are important for the control of hematopoiesis have been described, such as the Notch, Wingless-type (Wnt), and Hedgehog pathways [
16], which have been associated with self-renewal and maintenance of HSCs. Notch proteins are highly conserved receptors on the surface of the cell membrane that regulate the development of stem cells, and mutations in this receptor may cause leukemia [
17] and breast cancer [
18]. Activation of the Notch pathway is necessary to keep HSCs undifferentiated. This pathway is more active in HSCs and less active in differentiated cells. Inhibition of the Notch pathway potentiates the differentiation of HSCs and loss of the bone marrow reconstitution capacity of sub-lethally irradiated animals; thus, Notch has been used as a marker of undifferentiated HSCs [
19]. Wnt protein regulates several phenomena during fetal development, and this protein has been related to the self-renewal of stem cells [
20]. Hedgehog (Hh) protein has been described as regulating embryonic and adult stem cell activity. In mammals, three genes are known to be responsible for this protein—Sonic Hedgehog (SHh), Indian Hedgehog (IHh), and Desert Hedgehog (DHh) [
21,
22].
Soluble factors are also closely associated with the maintenance and regulation of the undifferentiated state of HSCs in the bone marrow of adults, in addition to regulating the proliferation and differentiation of this population. Stromal-cell-derived factor-1 (SDF-1/CXCL12) and its CXCR4 linker are activated to recruit endothelial progenitor cells (EPCs) and regulate HSCs [
2,
13,
23]. Other soluble factors act to promote the maintenance of HSCs in their niche; for example, the stem cell factor (SCF/Kit-ligand) and its c-Kit receptor (CD117) are both required by HSCs for their maintenance. SCF is an important soluble cytokine for hematopoiesis, and THE c-Kit receptor is expressed on the HSCs surface; altered forms of this receptor have been associated with several types of cancer [
24,
25]. Thrombopoietin (TPO) and its MPL ligand are also important soluble factors necessary for the maintenance of HSCs in their niche. TPO is a primary physiological regulator responsible for the stimulation of platelet production, a primary dominant factor and megakaryocytopoiesis stimulator. In addition, recent in vitro studies have shown that TPO alone or in combination with growth factors, such as a c-Kit ligand, IL-3, or even Flt-3, stimulates the proliferation of hematopoietic progenitor cells [
26,
27]. Many other factors also modulate the function of HSCs but are not necessarily required, such as angiogenin, angiopoietin-1, G-CSF, IL-6, and TGFβ, among others [
25].