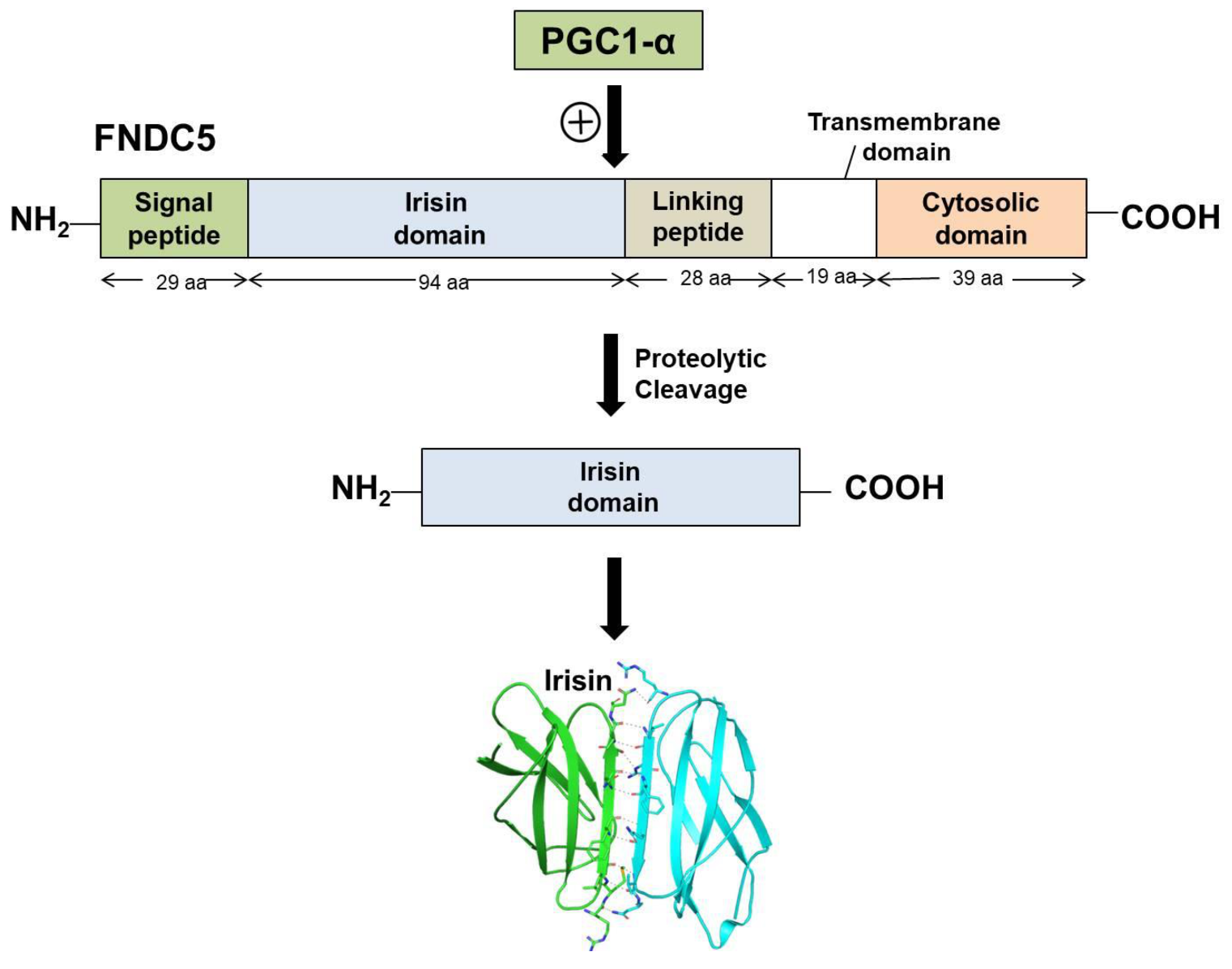
Irisin is a portion of the cell membrane protein known as FNDC5 (FNDC5 Fibronectin type III domain-containing protein 5). FNDC5 consists of a signal peptide, a fibronectin III domain, and a C-terminal domain. FNDC5 comprises 209 amino acid residues, having a signal sequence of 29 amino acids at the N-terminal end, followed by a 94-amino-acid residue fibronectin III (FNIII) 2 domains (irisin domain), a linking peptide comprising 28 amino acid residues, a 19-amino-acid residue transmembrane domain, and a cytoplasmic domain consisting of 39 amino acid residues.
- irisin
- structural insight
- therapeutic potential
- human pathophysiology
1. Biosynthesis and Secretion of Irisin
2. Structural Features and Signaling Pathways
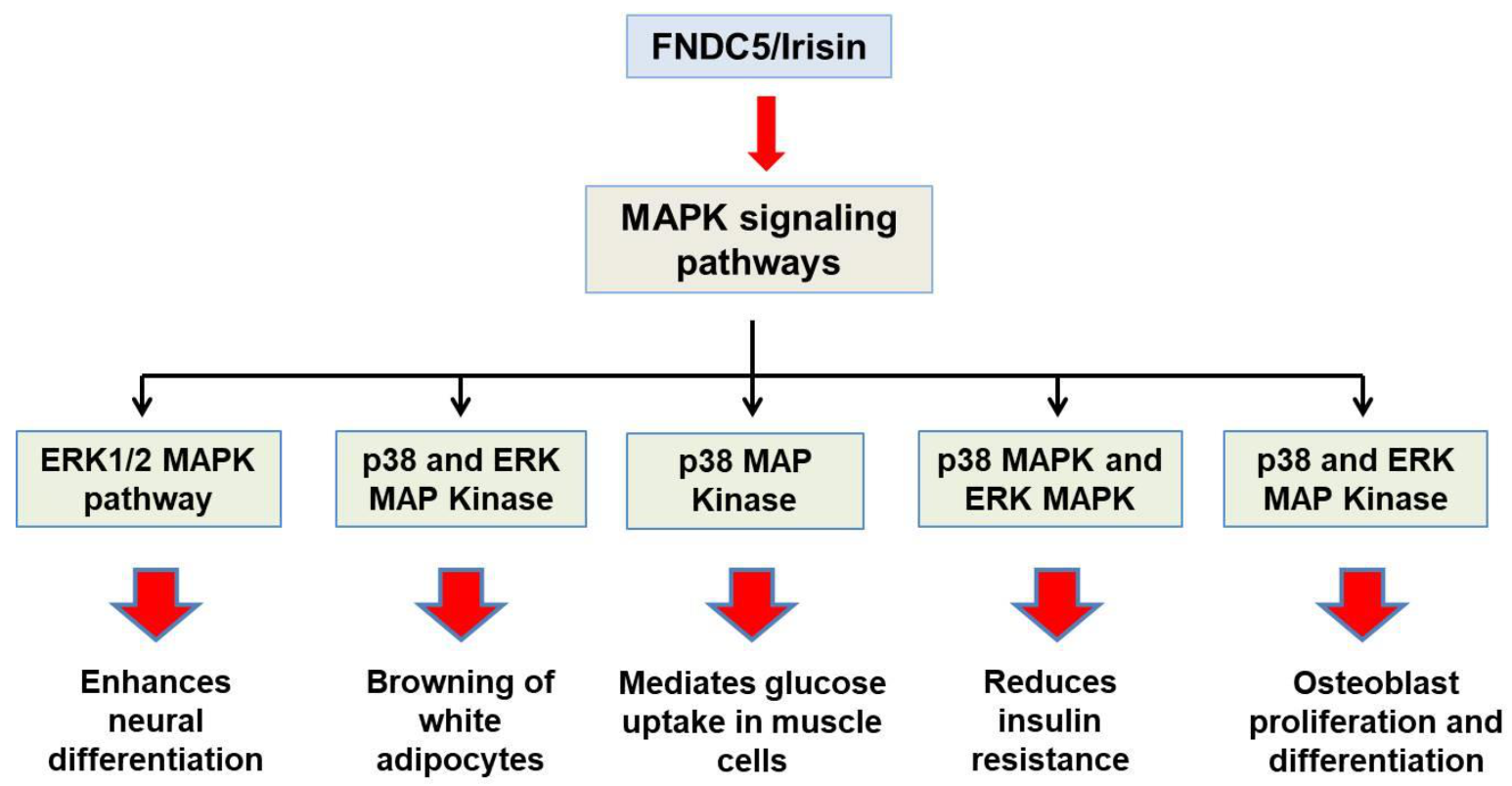
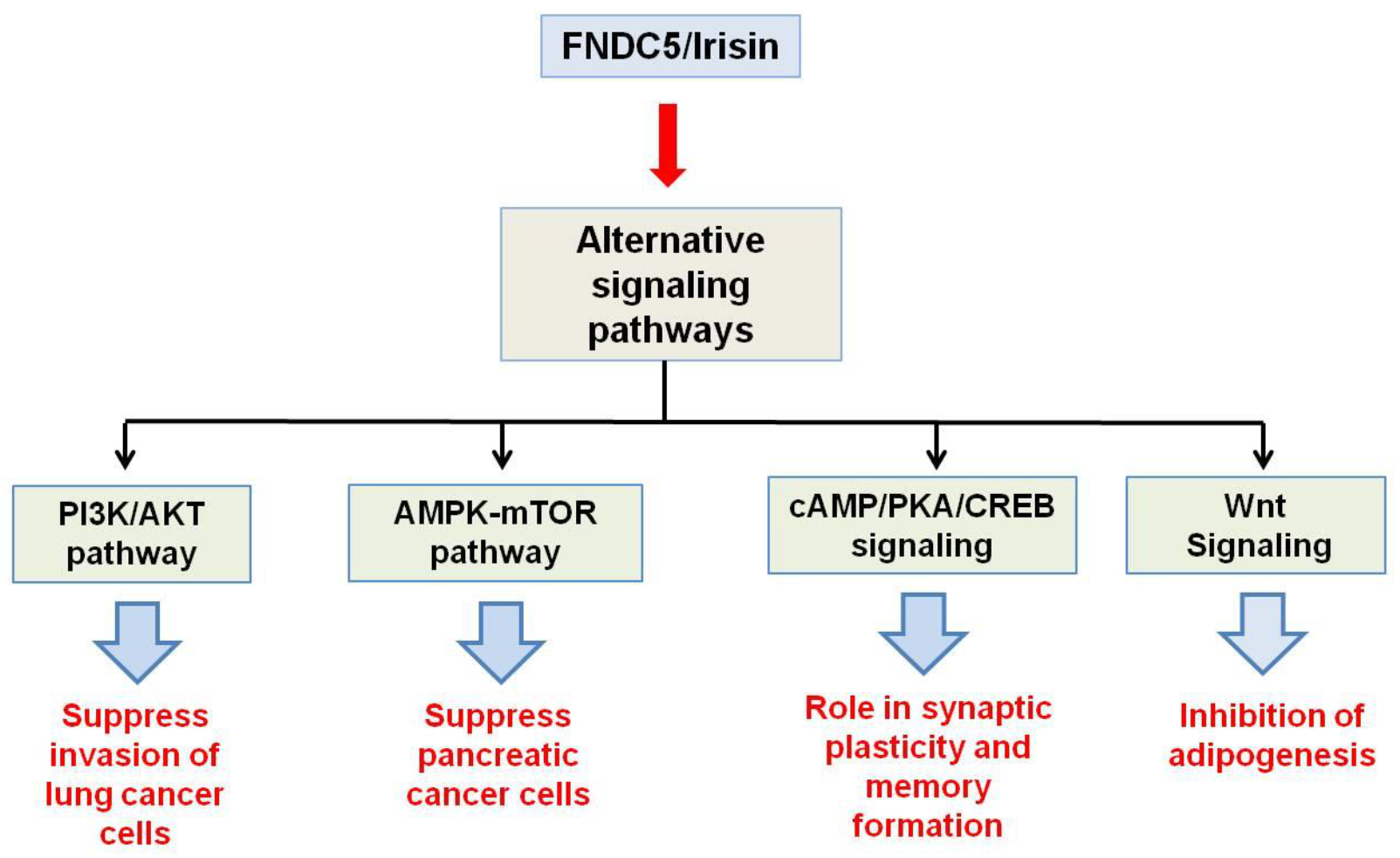
3. Irisin Receptor
4. Irisin in Obesity and Diabetes
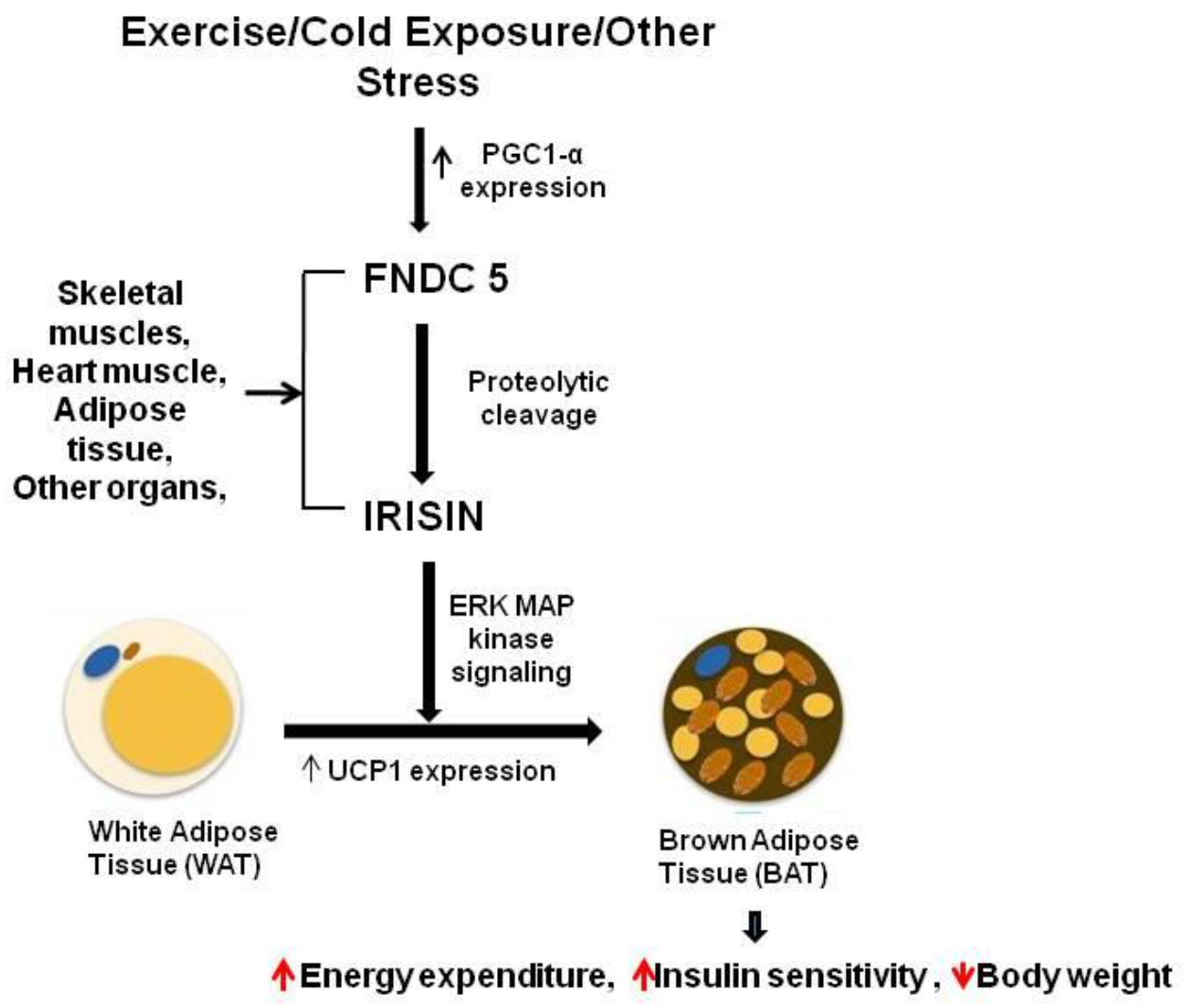
5. Irisin in the Nervous System
Abbreviations
FNDC5 Fibronectin type III domain-containing protein 5
BDNF Brain-derived neurotrophic factor
WAT White adipose tissue
BAT Brown adipose tissue
PGC1 α Peroxisome proliferator-activated receptor γ (PPAR-γ) coactivator 1-α
AMPK Adenosine monophosphate-activated protein kinase
PI3K Phosphatidylinositol 3-kinase
STAT3 Signal transducer and activator of transcription 3
MAPK Mitogen-activated protein kinase
ERK1/2 Extracellular signal-regulated kinase 1/2
cAMP Cyclic adenosine 3, 5- monophosphate
CREB cAMP response element binding
ATP Adenosine triphosphate
mTOR Mammalian target of rapamycin
References
- Korta, P.; Pocheć, E.; Mazur-Biały, A. Irisin as a multifunctional protein: Implications for health and certain diseases. Medicina 2019, 55, 485.
- Waseem, R.; Shamsi, A.; Mohammad, T.; Alhumaydhi, F.A.; Kazim, S.N.; Hassan, M.I.; Ahmad, F.; Islam, A. Multispectroscopic and Molecular Docking Insight into Elucidating the Interaction of Irisin with Rivastigmine Tartrate: A Combinational Therapy Approach to Fight Alzheimer’s Disease. ACS Omega 2021, 6, 7910–7921.
- Norheim, F.; Langleite, T.M.; Hjorth, M.; Holen, T.; Kielland, A.; Stadheim, H.K.; Gulseth, H.L.; Birkeland, K.I.; Jensen, J.; Drevon, C.A. The effects of acute and chronic exercise on PGC-1α, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014, 281, 739–749.
- Clin, A. The Relationship between High-Fat Diet and Fibronectin Type-III Domain-Containing Protein 5 mRNA Expression. Anatol. Clin. J. Med. Sci. 2018, 23, 1–5.
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F.; Ricart, W.; Fernández-Real, J.M. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778.
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468.
- Jedrychowski, M.P.; Wrann, C.D.; Paulo, J.A.; Gerber, K.K.; Szpyt, J.; Robinson, M.M.; Nair, K.S.; Gygi, S.P.; Spiegelman, B.M. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015, 22, 734–740.
- Raschke, S.; Elsen, M.; Gassenhuber, H.; Sommerfeld, M.; Schwahn, U.; Brockmann, B.; Jung, R.; Wisløff, U.; Tjønna, A.E.; Raastad, T. Evidence against a beneficial effect of irisin in humans. PLoS ONE 2013, 8, e73680.
- Vanin, E.F. Processed pseudogenes: Characteristics and evolution. Annu. Rev. Genet. 1985, 19, 253–272.
- Albrecht, E.; Norheim, F.; Thiede, B.; Holen, T.; Ohashi, T.; Schering, L.; Lee, S.; Brenmoehl, J.; Thomas, S.; Drevon, C.A. Irisin–a myth rather than an exercise-inducible myokine. Sci. Rep. 2015, 5, 8889.
- Lee, P.; Linderman, J.D.; Smith, S.; Brychta, R.J.; Wang, J.; Idelson, C.; Perron, R.M.; Werner, C.D.; Phan, G.Q.; Kammula, U.S. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014, 19, 302–309.
- Ruan, Q.; Zhang, L.; Ruan, J.; Zhang, X.; Chen, J.; Ma, C.; Yu, Z. Detection and quantitation of irisin in human cerebrospinal fluid by tandem mass spectrometry. Peptides 2018, 103, 60–64.
- Ruan, Q.; Huang, Y.; Yang, L.; Ruan, J.; Gu, W.; Zhang, X.; Zhang, Y.; Zhang, W.; Yu, Z. The effects of both age and sex on irisin levels in paired plasma and cerebrospinal fluid in healthy humans. Peptides 2019, 113, 41–51.
- Colaianni, G.; Errede, M.; Sanesi, L.; Notarnicola, A.; Celi, M.; Zerlotin, R.; Storlino, G.; Pignataro, P.; Oranger, A.; Pesce, V. Irisin correlates positively with BMD in a cohort of older adult patients and downregulates the senescent marker p21 in osteoblasts. J. Bone Miner. Res. 2021, 36, 305–314.
- Colaianni, G.; Storlino, G.; Sanesi, L.; Colucci, S.; Grano, M. Myokines and osteokines in the pathogenesis of muscle and bone diseases. Curr. Osteoporos. Rep. 2020, 18, 401–407.
- Young, M.F.; Valaris, S.; Wrann, C.D. A role for FNDC5/Irisin in the beneficial effects of exercise on the brain and in neurodegenerative diseases. Prog. Cardiovasc. Dis. 2019, 62, 172–178.
- Schumacher, M.A.; Chinnam, N.; Ohashi, T.; Shah, R.S.; Erickson, H.P. The structure of irisin reveals a novel intersubunit β-sheet fibronectin type III (FNIII) dimer: Implications for receptor activation. J. Biol. Chem. 2013, 288, 33738–33744.
- Korta, P.; Pocheć, E. Glycosylation of thyroid-stimulating hormone receptor. Endokrynol. Pol. 2019, 70, 86–100.
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes 2014, 63, 514–525.
- Erickson, K.I.; Weinstein, A.M.; Lopez, O.L. Physical activity, brain plasticity, and Alzheimer’s disease. Arch. Med. Res. 2012, 43, 615–621.
- Li, X.; Duan, H.; Liu, Q.; Umar, M.; Luo, W.; Yang, X.; Zhu, J.; Li, M. Construction of a Pichia pastoris strain efficiently secreting irisin and assessment of its bioactivity in HepG2 cells. Int. J. Biol. Macromol. 2019, 124, 60–70.
- Rabiee, F.; Lachinani, L.; Ghaedi, S.; Nasr-Esfahani, M.H.; Megraw, T.L.; Ghaedi, K. New insights into the cellular activities of Fndc5/Irisin and its signaling pathways. Cell Biosci. 2020, 10, 1–10.
- Liu, J.; Song, N.; Huang, Y.; Chen, Y. Irisin inhibits pancreatic cancer cell growth via the AMPK-mTOR pathway. Sci. Rep. 2018, 8, 1–10.
- Shao, L.; Li, H.; Chen, J.; Song, H.; Zhang, Y.; Wu, F.; Wang, W.; Zhang, W.; Wang, F.; Li, H. Irisin suppresses the migration, proliferation, and invasion of lung cancer cells via inhibition of epithelial-to-mesenchymal transition. Biochem. Biophys. Res. Commun. 2017, 485, 598–605.
- Lourenco, M.V.; Frozza, R.L.; de Freitas, G.B.; Zhang, H.; Kincheski, G.C.; Ribeiro, F.C.; Gonçalves, R.A.; Clarke, J.R.; Beckman, D.; Staniszewski, A. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat. Med. 2019, 25, 165.
- Ma, E.B.; Sahar, N.E.; Jeong, M.; Huh, J.Y. Irisin exerts inhibitory effect on adipogenesis through regulation of Wnt signaling. Front. Physiol. 2019, 10, 1085.
- Kim, H.; Wrann, C.D.; Jedrychowski, M.; Vidoni, S.; Kitase, Y.; Nagano, K.; Zhou, C.; Chou, J.; Parkman, V.-J.A.; Novick, S.J. Irisin mediates effects on bone and fat via αV integrin receptors. Cell 2018, 175, 1756–1768.
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Zecca, C.; Dell’Abate, M.T.; Buccoliero, C.; Logroscino, G.; Colucci, S.; Grano, M. FNDC5/Irisin System in Neuroinflammation and Neurodegenerative Diseases: Update and Novel Perspective. Int. J. Mol. Sci. 2021, 22, 1605.
- Rosen, E.D.; Spiegelman, B.M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006, 444, 847–853.
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691.
- Luo, Y.; Qiao, X.; Ma, Y.; Deng, H.; Xu, C.C.; Xu, L. Disordered metabolism in mice lacking irisin. Sci. Rep. 2020, 10, 1–10.
- Park, K.H.; Zaichenko, L.; Brinkoetter, M.; Thakkar, B.; Sahin-Efe, A.; Joung, K.E.; Tsoukas, M.A.; Geladari, E.V.; Huh, J.Y.; Dincer, F. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J. Clin. Endocrinol. Metab. 2013, 98, 4899–4907.
- Liu, S.; Du, F.; Li, X.; Wang, M.; Duan, R.; Zhang, J.; Wu, Y.; Zhang, Q. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic β cells. PLoS ONE 2017, 12, e0175498.
- Chen, N.; Li, Q.; Liu, J.; Jia, S. Irisin, an exercise-induced myokine as a metabolic regulator: An updated narrative review. Diabetes/Metab. Res. Rev. 2016, 32, 51–59.
- Choi, Y.-K.; Kim, M.-K.; Bae, K.H.; Seo, H.-A.; Jeong, J.-Y.; Lee, W.-K.; Kim, J.-G.; Lee, I.-K.; Park, K.-G. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 100, 96–101.
- Liu, J.-J.; Wong, M.D.; Toy, W.C.; Tan, C.S.; Liu, S.; Ng, X.W.; Tavintharan, S.; Sum, C.F.; Lim, S.C. Lower circulating irisin is associated with type 2 diabetes mellitus. J. Diabetes Its Complicat. 2013, 27, 365–369.
- Zhu, D.; Wang, H.; Zhang, J.; Zhang, X.; Xin, C.; Zhang, F.; Lee, Y.; Zhang, L.; Lian, K.; Yan, W. Irisin improves endothelial function in type 2 diabetes through reducing oxidative/nitrative stresses. J. Mol. Cell. Cardiol. 2015, 87, 138–147.
- So, J.H.; Huang, C.; Ge, M.; Cai, G.; Zhang, L.; Lu, Y.; Mu, Y. Intense exercise promotes adult hippocampal neurogenesis but not spatial discrimination. Front. Cell. Neurosci. 2017, 11, 13.
- Piya, M.K.; Harte, A.L.; Sivakumar, K.; Tripathi, G.; Voyias, P.D.; James, S.; Sabico, S.; Al-Daghri, N.M.; Saravanan, P.; Barber, T.M. The identification of irisin in human cerebrospinal fluid: Influence of adiposity, metabolic markers, and gestational diabetes. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E512–E518.
- Novelle, M.G.; Contreras, C.; Romero-Picó, A.; López, M.; Diéguez, C. Irisin, two years later. Int. J. Endocrinol. 2013, 2013, 1–8.
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659.
- Moon, H.-S.; Dincer, F.; Mantzoros, C.S. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19-7 hippocampal cell lines. Metabolism 2013, 62, 1131–1136.
- Pesce, M.; Ballerini, P.; Paolucci, T.; Puca, I.; Farzaei, M.H.; Patruno, A. Irisin and autophagy: First update. Int. J. Mol. Sci. 2020, 21, 7587.
- Xin, T.; Lu, C. Irisin activates Opa1-induced mitophagy to protect cardiomyocytes against apoptosis following myocardial infarction. Aging 2020, 12, 4474.
