The developments within the topic of biomaterials has taken hold of researchers due to the mounting concern of current environmental pollution as well as scarcity resources. Amongst all compatible biomaterials, polycaprolactone (PCL) is deemed to be a great potential biomaterial, especially to the tissue engineering sector, due to its advantages, including its biocompatibility and low bioactivity exhibition. The commercialization of PCL is deemed as infant technology despite of all its advantages. This contributed to the disadvantages of PCL, including expensive, toxic, and complex.
- polycaprolactone
- green biocomposites
- hybrid biocomposites
- mechanical properties
- thermal properties
1. Introduction
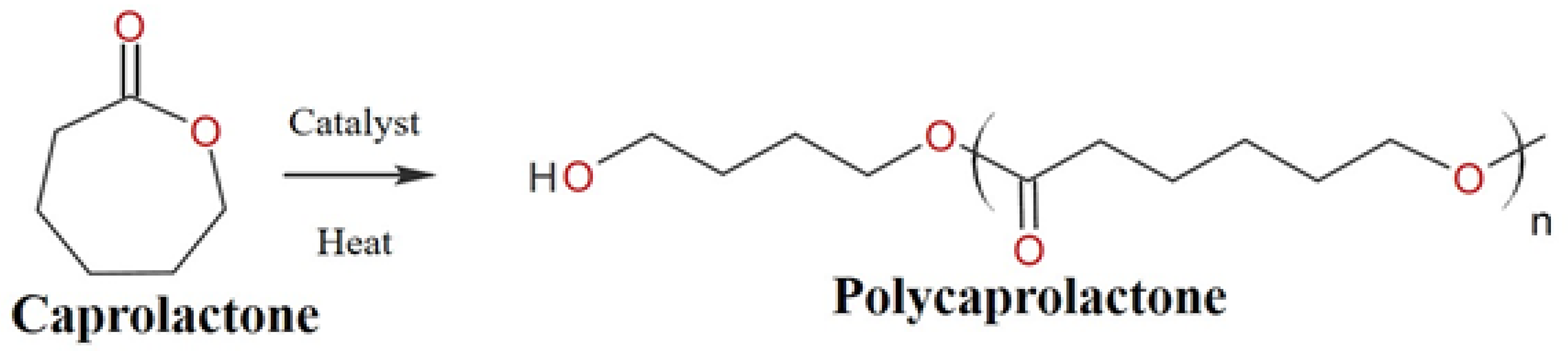
Among several types of biopolymers, polycaprolactone (PCL) has received a lot of attention due to its several advantages, such as its biodegradability, high strength, and biocompatibility. It can withstand water, oil, solvents, and chlorine. PCL is a semicrystalline ester polymer that is derived from ring-opening polymerization of ε-caprolactone monomers, as shown in Figure 1. PCL consists of a glass transition temperature (Tg) of around 60 °C and a melting point ranging between 59–64 °C, dictated by the crystalline nature of PCL which enables easy formability at relatively low temperatures [1].
| Molecular Weight | Melting Point, °C | Tensile Stress, N/m2 | Elongation at Break, % |
|---|---|---|---|
| 37,000 | 58–60 | 1.37 × 107 | 660 |
| 50,000 | 58–60 | 3.53 × 107 | 800 |
| 80,000 | 60–62 | 5.69 × 107 | 900 |
| Polyester | Degradation By-Products (pKa) | In Vivo Degradation Rate | Degradation Mechanism |
|---|---|---|---|
| PCL | Caproic acid (4.88) | 50% in 4 years 1% in 6 months |
Hydrolytic degradation |
| PLA | Lactic acid (3.85) Lactic acid (3.08) |
| Advantages | Disadvantages | ||
|---|---|---|---|
| High biocompatibility | Adheres poorly to cells | ||
| 50% in 1–2 years 98% in 12 months 100% in >12 months 100% in 12–16 month | Hydrolysis through the action of enzymes | ||
| Highly biodegradable | Toxic solvent | PGA | Glycolic acid (3.83) |
| Great electrospinning properties | 100% in 2–3 month | 100% in 6–12 months |
Both enzymatic and non-enzymatic hydrolysis |
| Low melting point | |
| Long biodegradable time | Complex and expensive production |
| High material purity |
2. Applications of Polycaprolactone-Based Biocomposites
3. Conclusions
References
- Sisson, A.L.; Ekinci, D.; Lendlein, A. The contemporary role of ε-caprolactone chemistry to create advanced polymer architectures. Polymer 2013, 54, 4333–4350.
- Mohamed, R.M.; Yusoh, K. A Review on the Recent Research of Polycaprolactone (PCL). Adv. Mater. Res. 2015, 1134, 249–255.
- Jiang, L.; Zhang, J. 7 Biodegradable and Biobased Polymers, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780323390408.
- Nevoralová, M.; Koutný, M.; Ujčić, A.; Starý, Z.; Šerá, J.; Vlková, H.; Šlouf, M.; Fortelný, I.; Kruliš, Z. Structure Characterization and Biodegradation Rate of Poly(ε-caprolactone)/Starch Blends. Front. Mater. 2020, 7, 141.
- Acik, G. Bio-based Poly(ɛ-caprolactone) from Soybean-Oil Derived Polyol via Ring-Opening Polymerization. J. Polym. Environ. 2020, 28, 668–675.
- Johansson, C.; Bras, J.; Mondragon, I.; Nechita, P.; Plackett, D.; Šimon, P.; Svetec, D.G.; Virtanen, S.; Baschetti, M.G.; Breen, C.; et al. Renewable fibers and bio-based materials for packaging applications—A review of recent developments. BioResources 2012, 7, 2506–2552.
- Woodruff, M.A.; Hutmacher, D.W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010, 35, 1217–1256.
- Patr, T.; Glória, A. Mechanical and biological behaviour of PCL and PCL/PLA scaffolds for tissue engineering applications. Chem. Eng. Trans. 2013, 32, 1645–1650.
- Miller, K.; Hsu, J.E.; Soslowsky, L.J. Materials in Tendon and Ligament Repair; Elsevier Ltd.: Amsterdam, The Netherlands, 2011; Volume 6, ISBN 9780080552941.
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893.
- Todea, A.; Bîtcan, I.; Aparaschivei, D.; Păușescu, I.; Badea, V.; Péter, F.; Gherman, V.D.; Rusu, G.; Nagy, L.; Kéki, S. Biodegradable Oligoesters of ε-Caprolactone and 5-Hydroxymethyl-2-Furancarboxylic Acid Synthesized by Immobilized Lipases. Polymer 2019, 11, 1402.
- Manivasagam, G.; Reddy, A.; Sen, D.; Nayak, S.; Mathew, M.T.; Rajamanikam, A. Dentistry: Restorative and Regenerative Approaches; Elsevier: Amsterdam, The Netherlands, 2019; Volume 1–3, ISBN 9780128051443.
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical applications of biodegradable polyesters. Polymers 2016, 8, 20.
- Arunagiri, V.; Prasannan, A.; Udomsin, J.; Lai, J.-Y.; Wang, C.-F.; Hong, P.-D.; Tsai, H.C. Facile fabrication of eco-friendly polycaprolactone (PCL)/Poly-D, L-Lactic acid (PDLLA) modified melamine sorbent for oil-spill cleaning and water/oil (W/O) emulsion separation. Sep. Purif. Technol. 2021, 259, 118081.
- Kim, S.; Gwon, Y.; Park, S.; Kim, W.; Jeon, Y.; Han, T.; Jeong, H.E.; Kim, J. Synergistic effects of gelatin and nanotopographical patterns on biomedical PCL patches for enhanced mechanical and adhesion properties. J. Mech. Behav. Biomed. Mater. 2021, 114, 104167.
- Valerini, D.; Tammaro, L.; Vitali, R.; Guillot, G.; Rinaldi, A. Sputter-Deposited Ag Nanoparticles on Electrospun PCL Scaffolds: Morphology, Wettability and Antibacterial Activity. Coatings 2021, 11, 345.
- Ebrahimifar, M.; Taherimehr, M. Evaluation of in-vitro drug release of polyvinylcyclohexane carbonate as a CO2-derived degradable polymer blended with PLA and PCL as drug carriers. J. Drug Deliv. Sci. Technol. 2021, 63, 102491.
- El-Naggar, M.E.; Shalaby, E.S.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. Technol. 2021, 15, 3447–3460.
- Herrero-Herrero, M.; Alberdi-Torres, S.; González-Fernández, M.L.; Vilariño-Feltrer, G.; Rodríguez-Hernández, J.C.; Vallés-Lluch, A.; Villar-Suárez, V. Influence of chemistry and fiber diameter of electrospun PLA, PCL and their blend membranes, intended as cell supports, on their biological behavior. Polym. Test. 2021, 103, 107364.
- Doganci, M.D. Effects of star-shaped PCL having different numbers of arms on the mechanical, morphological, and thermal properties of PLA/PCL blends. J. Polym. Res. 2021, 28, 11.
- Ouled Ltaief, A.; Ghorbel, N.; Benhamou, K.; Arous, M.; Kaddami, H.; Kallel, A. Impact of cellulose nanocrystals reinforcement on molecular dynamics and dielectric properties of PCL-based polyurethane. Polym. Compos. 2021, 42, 2737–2750.
- dos Santos Filho, E.A.; Siqueira, D.D.; Araújo, E.M.; Luna, C.B.B.; de Medeiros, E.P. The Impact of the Macaíba Components Addition on the Biodegradation Acceleration of Poly (Ɛ-Caprolactone) (PCL). J. Polym. Environ. 2021, 1–18.
- Reis, R.S.; Souza, D. de H.S.; Marques, M. de F.V.; da Luz, F.S.; Monteiro, S.N. Novel bionanocomposite of polycaprolactone reinforced with steam-exploded microfibrillated cellulose modified with ZnO. J. Mater. Res. Technol. 2021, 13, 1324–1335.
- Ramamoorthy, R.; Andiappan, M.; Muthalagu, M. Preparation and characterization of Terminalia bellerica loaded PCL nanofibrous mats for biomedical applications. Mater. Today Proc. 2021, 45, 7247–7252.
- Balan, R.; Gayathri, V. In-vitro and antibacterial activities of novel POT/TiO2/PCL composites for tissue engineering and biomedical applications. Polym. Bull. 2021, 1–18.
- El Fawal, G.; Hong, H.; Mo, X.; Wang, H. Fabrication of scaffold based on gelatin and polycaprolactone (PCL) for wound dressing application. J. Drug Deliv. Sci. Technol. 2021, 63, 102501.
- Ma, S.; Jiang, Z.; Wang, M.; Zhang, L.; Liang, Y.; Zhang, Z.; Ren, L.; Ren, L. 4D printing of PLA/PCL shape memory composites with controllable sequential deformation. Bio-Design Manuf. 2021, 4, 867–878.
- Al-Kaabi, W.J.; Albukhaty, S.; Al-Fartosy, A.J.M.; Al-Karagoly, H.K.; Al-Musawi, S.; Sulaiman, G.M.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Development of Inula graveolens (L.) Plant Extract Electrospun/Polycaprolactone Nanofibers: A Novel Material for Biomedical Application. Appl. Sci. 2021, 11, 828.
- Hajiali, F.; Tajbakhsh, S.; Shojaei, A. Fabrication and Properties of Polycaprolactone Composites Containing Calcium Phosphate-Based Ceramics and Bioactive Glasses in Bone Tissue Engineering: A Review. Polym. Rev. 2018, 58, 164–207.
- Moers-Carpi, M.M.; Sherwood, S. Polycaprolactone for the Correction of Nasolabial Folds: A 24-Month, Prospective, Randomized, Controlled Clinical Trial. Dermatol. Surg. 2013, 39, 457–463.
- Hao, Y.; Chen, Y.; He, X.; Yang, F.; Han, R.; Yang, C.; Li, W.; Qian, Z. Near-infrared responsive 5-fluorouracil and indocyanine green loaded MPEG-PCL nanoparticle integrated with dissolvable microneedle for skin cancer therapy. Bioact. Mater. 2020, 5, 542–552.
- Peng, W.; Jiang, X.; Zhu, Y.; Omari-Siaw, E.; Deng, W.; Yu, J.; Xu, X.; Zhang, W. Oral delivery of capsaicin using MPEG-PCL nanoparticles. Acta Pharmacol. Sin. 2015, 36, 139–148.
- Kim, J.A.; Van Abel, D. Neocollagenesis in human tissue injected with a polycaprolactone-based dermal filler. J. Cosmet. Laser Ther. 2015, 17, 99–101.
- Christen, M.-O.; Vercesi, F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48.
- de Melo, F.; Carrijo, A.; Hong, K.; Trumbic, B.; Vercesi, F.; Waldorf, H.A.; Zenker, S. Minimally Invasive Aesthetic Treatment of the Face and Neck Using Combinations of a PCL-Based Collagen Stimulator, PLLA/PLGA Suspension Sutures, and Cross-Linked Hyaluronic Acid. Clin. Cosmet. Investig. Dermatol. 2020, 13, 333–344.
- de Melo, F.; Nicolau, P.; Piovano, L.; Lin, S.-L.; Baptista-Fernandes, T.; King, M.I.; Camporese, A.; Hong, K.; Khattar, M.; Christen, M.-O. Recommendations for volume augmentation and rejuvenation of the face and hands with the new generation polycaprolactone-based collagen stimulator (Ellansé®). Clin. Cosmet. Investig. Dermatol. 2017, 10, 431–440.
- Morera Serna, E.; Serna Benbassat, M.; Terré Falcón, R.; Murillo Martín, J. Anatomy and Aging of the Perioral Region. Facial Plast. Surg. 2021, 37, 176–193.
- Goodwin, P. Collagen stimulation with a range of polycaprolactone dermal fillers. J. Aesthetic Nurs. 2018, 7, 22–28.
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials 2005, 26, 4139–4147.
- Won, J.-Y.; Park, C.-Y.; Bae, J.-H.; Ahn, G.; Kim, C.; Lim, D.-H.; Cho, D.-W.; Yun, W.-S.; Shim, J.-H.; Huh, J.-B. Evaluation of 3D printed PCL/PLGA/ β -TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed. Mater. 2016, 11, 055013.
- Ji, W.; Yang, F.; Ma, J.; Bouma, M.J.; Boerman, O.C.; Chen, Z.; van den Beucken, J.J.J.P.; Jansen, J.A. Incorporation of stromal cell-derived factor-1α in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials 2013, 34, 735–745.
- Shim, J.-H.; Yoon, M.-C.; Jeong, C.-M.; Jang, J.; Jeong, S.-I.; Cho, D.-W.; Huh, J.-B. Efficacy of rhBMP-2 loaded PCL/PLGA/ β -TCP guided bone regeneration membrane fabricated by 3D printing technology for reconstruction of calvaria defects in rabbit. Biomed. Mater. 2014, 9, 065006.
- Türkkan, S.; Pazarçeviren, A.E.; Keskin, D.; Machin, N.E.; Duygulu, Ö.; Tezcaner, A. Nanosized CaP-silk fibroin-PCL-PEG-PCL/PCL based bilayer membranes for guided bone regeneration. Mater. Sci. Eng. C 2017, 80, 484–493.
- Ren, K.; Wang, Y.; Sun, T.; Yue, W.; Zhang, H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C 2017, 78, 324–332.
- Castro, A.G.B.; Diba, M.; Kersten, M.; Jansen, J.A.; van den Beucken, J.J.J.P.; Yang, F. Development of a PCL-silica nanoparticles composite membrane for Guided Bone Regeneration. Mater. Sci. Eng. C 2018, 85, 154–161.
- Ahmed, F.; Discher, D.E. Self-porating polymersomes of PEG–PLA and PEG–PCL: Hydrolysis-triggered controlled release vesicles. J. Control. Release 2004, 96, 37–53.
- Köthe, T.; Martin, S.; Reich, G.; Fricker, G. Dual asymmetric centrifugation as a novel method to prepare highly concentrated dispersions of PEG-b-PCL polymersomes as drug carriers. Int. J. Pharm. 2020, 579, 119087.
- Zwawi, M. A Review on Natural Fiber Bio-Composites, Surface Modifications and Applications. Molecules 2021, 26, 404.
- Grossen, P.; Witzigmann, D.; Sieber, S.; Huwyler, J. PEG-PCL-based nanomedicines: A biodegradable drug delivery system and its application. J. Control. Release 2017, 260, 46–60.
- Rai, B.; Teoh, S.H.; Hutmacher, D.W.; Cao, T.; Ho, K.H. Novel PCL-based honeycomb scaffolds as drug delivery systems for rhBMP-2. Biomaterials 2005, 26, 3739–3748.
- Nabid, M.R.; Tabatabaei Rezaei, S.J.; Sedghi, R.; Niknejad, H.; Entezami, A.A.; Oskooie, H.A.; Heravi, M.M. Self-assembled micelles of well-defined pentaerythritol-centered amphiphilic A4B8 star-block copolymers based on PCL and PEG for hydrophobic drug delivery. Polymer 2011, 52, 2799–2809.
- Holländer, J.; Genina, N.; Jukarainen, H.; Khajeheian, M.; Rosling, A.; Mäkilä, E.; Sandler, N. Three-Dimensional Printed PCL-Based Implantable Prototypes of Medical Devices for Controlled Drug Delivery. J. Pharm. Sci. 2016, 105, 2665–2676.
- Gong, C.; Shi, S.; Wu, L.; Gou, M.; Yin, Q.; Guo, Q.; Dong, P.; Zhang, F.; Luo, F.; Zhao, X.; et al. Biodegradable in situ gel-forming controlled drug delivery system based on thermosensitive PCL–PEG–PCL hydrogel. Part 2: Sol–gel–sol transition and drug delivery behavior. Acta Biomater. 2009, 5, 3358–3370.
- Coombes, A.G.A.; Rizzi, S.C.; Williamson, M.; Barralet, J.E.; Downes, S.; Wallace, W.A. Precipitation casting of polycaprolactone for applications in tissue engineering and drug delivery. Biomaterials 2004, 25, 315–325.
- Chang, C.; Wei, H.; Quan, C.-Y.; Li, Y.-Y.; Liu, J.; Wang, Z.-C.; Cheng, S.-X.; Zhang, X.-Z.; Zhuo, R.-X. Fabrication of thermosensitive PCL-PNIPAAm-PCL triblock copolymeric micelles for drug delivery. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3048–3057.
- Danafar, H. MPEG–PCL copolymeric nanoparticles in drug delivery systems. Cogent Med. 2016, 3, 1142411.
- Zhang, J.; Zhao, S.; Zhu, M.; Zhu, Y.; Zhang, Y.; Liu, Z.; Zhang, C. 3D-printed magnetic Fe3O4 /MBG/PCL composite scaffolds with multifunctionality of bone regeneration, local anticancer drug delivery and hyperthermia. J. Mater. Chem. B 2014, 2, 7583–7595.
- Deng, H.; Dong, A.; Song, J.; Chen, X. Injectable thermosensitive hydrogel systems based on functional PEG/PCL block polymer for local drug delivery. J. Control. Release 2019, 297, 60–70.
- Zhao, P.; Liu, L.; Feng, X.; Wang, C.; Shuai, X.; Chen, Y. Molecular Nanoworm with PCL Core and PEO Shell as a Non-spherical Carrier for Drug Delivery. Macromol. Rapid Commun. 2012, 33, 1351–1355.
- Dash, T.K.; Konkimalla, V.B. Polymeric Modification and Its Implication in Drug Delivery: Poly-ε-caprolactone (PCL) as a Model Polymer. Mol. Pharm. 2012, 9, 2365–2379.
- Hosseinkazemi, H.; Biazar, E.; Bonakdar, S.; Ebadi, M.-T.; Shokrgozar, M.-A.; Rabiee, M. Modification of PCL Electrospun Nanofibrous Mat With Calendula officinalis Extract for Improved Interaction With Cells. Int. J. Polym. Mater. Polym. Biomater. 2015, 64, 459–464.
- Hosseini, Y.; Emadi, R.; Kharaziha, M. Surface modification of PCL-diopside fibrous membrane via gelatin immobilization for bone tissue engineering. Mater. Chem. Phys. 2017, 194, 356–366.
- Kweon, H. A novel degradable polycaprolactone networks for tissue engineering. Biomaterials 2003, 24, 801–808.
- Siddiqui, N.; Asawa, S.; Birru, B.; Baadhe, R.; Rao, S. PCL-Based Composite Scaffold Matrices for Tissue Engineering Applications. Mol. Biotechnol. 2018, 60, 506–532.
- Kundu, J.; Shim, J.-H.; Jang, J.; Kim, S.-W.; Cho, D.-W. An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering. J. Tissue Eng. Regen. Med. 2015, 9, 1286–1297.
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA Scaffolds for Tissue Engineering. Procedia CIRP 2013, 5, 110–114.
- Gautam, S.; Dinda, A.K.; Mishra, N.C. Fabrication and characterization of PCL/gelatin composite nanofibrous scaffold for tissue engineering applications by electrospinning method. Mater. Sci. Eng. C 2013, 33, 1228–1235.
- Boia, R.; Dias, P.A.N.; Martins, J.M.; Galindo-Romero, C.; Aires, I.D.; Vidal-Sanz, M.; Agudo-Barriuso, M.; de Sousa, H.C.; Ambrósio, A.F.; Braga, M.E.M.; et al. Porous poly(ε-caprolactone) implants: A novel strategy for efficient intraocular drug delivery. J. Control. Release 2019, 316, 331–348.
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Drug release and biodegradability of electrospun cellulose nanocrystal reinforced polycaprolactone. Mater. Sci. Eng. C 2019, 94, 929–937.
