Metabolic syndrome is a cluster of metabolic indicators that increase the risk of diabetes and cardiovascular diseases. Visceral obesity and factors derived from altered adipose tissue, adipokines, play critical roles in the development of metabolic syndrome. Although the adipokines leptin and adiponectin improve insulin sensitivity, others contribute to the development of glucose intolerance, including visfatin, fetuin-A, resistin, and plasminogen activator inhibitor-1 (PAI-1). Leptin and adiponectin increase fatty acid oxidation, prevent foam cell formation, and improve lipid metabolism, while visfatin, fetuin-A, PAI-1, and resistin have pro-atherogenic properties.
- adipokine
- metabolic syndrome
- glucose intolerance
- lipid metabolism
1. Introduction
Metabolic syndrome is a combination of interrelated conditions that often occur together, including obesity, insulin resistance, glucose intolerance, hypertension, and dyslipidemia [1][3]. Metabolic syndrome is diagnosed as the presence of at least three of the following five characteristics: high waist–hip ratio, high blood pressure, elevated blood sugar level, increased triglycerides (TGs), and low high-density lipoprotein (HDL) cholesterol [2][4]. Metabolic syndrome is important because of its association with an increasing prevalence of diabetes and a higher risk of cardiovascular events such as heart disease and stroke, which have become major public health issues [3][5]. Dysregulation of certain adipokines can promote pathogenic conditions associated with obesity, lipid accumulation, and insulin resistance. These increase the risk of atherosclerosis [4][6].
2. Adipose Tissue as a Critical Endocrine Organ Causing Metabolic Syndrome
3. Dysregulation of Adipokines in Metabolic Syndrome
3.1. Leptin
3.2. Adiponectin
Adiponectin is a 30 kDa protein that originates from adipose tissue. Adiponectin exists as multimers in plasma and has three major oligomeric forms combined with its collagen domain: a low-molecular-weight trimer, a middle-molecular-weight hexamer, and high-molecular-weight (HMW) 12- to 18-mers [15][70]. HMW adiponectin is a superior biomarker associated with protection against metabolic syndrome as the most potent form in the activation of AMP kinase [16][71].3.3. Visfatin
Visfatin is a 52 kDa cytokine that functions like insulin and is expressed in various organs including skeletal muscle, liver, lymphocytes, and adipose tissue [17][18][86,87]. Visfatin was formerly known as NAMPT (or pre-B-colony-enhancing factor) and is a rate-limiting enzyme that converts nicotinamide to nicotinamide mononucleotide [17][18][86,87]. Visfatin is also released from visceral adipose tissue, predominantly from macrophages rather than from adipocytes [19][88].3.4. Fetuin-A
Fetuin-A, a 64 kDa glycoprotein also known as α2-Heremans–Schmid glycoprotein (AHSG), is mainly secreted from the liver and adipose tissue. Fetuin-A contributes to macrophage migration into adipose tissue [20][32] and augments the expression of proinflammatory cytokines such as IL-6 and TNF-α, while reducing adiponectin expression [21][95].3.5. Plasminogen Activator Inhibitor-1 (PAI-1)
PAI-1, a physiological inhibitor of plasminogen activators and vitronectin, is synthesized in adipose tissue.3.6. Omentin-1
Omentin, also known as Intelectin-1, is a secretory glycoprotein that is highly and selectively expressed in visceral adipose tissue relative to subcutaneous adipose tissue. Both the omentin-1 gene and omentin-2 gene, a homolog which shares an 83% amino acid identity with omentin-1, are located in the chromosome 1q22-q23 region, which has been previously linked to type 2 diabetes [22][45].3.7. Lipocalin-2
Lipocalin-2 (LCN-2, also called neutrophil gelatinase-associated lipocalin) is a 25 kDa protein that plays a role in the innate immune response to bacterial infection. LCN-2 is expressed in various sites such as liver, kidney, brain, lung, and adipocytes [22][45].4. Molecular and Cellular Crosstalk in Central Obesity and Metabolic Syndrome
Obesity has generally been considered a risk factor for metabolic and cardiovascular diseases for decades. However, recently, the paradox of obesity has been highlighted from a new perspective in which the location of fat accumulation is the problem rather than the total amount of fat [23][122]. There are two types of adipose tissue, white and brown, which perform different functions [24][123]. In humans, white adipose tissue consists mainly of a central intra-abdominal component (visceral adipose tissue) associated with increased metabolic risk, whereas subcutaneous adipose tissue has a protective effect on energy homeostasis and cardiovascular health [25][26][124,125]. A previous study showed that human subcutaneous adipose tissue contains larger adipocytes, is less infiltrated by CD68+ and M1-activated cells, and expresses higher levels of cardioprotective adipokines such as adiponectin [27][126]. Additionally, intraperitoneal implantation of subcutaneous adipose tissue in obese mice prevented glucose intolerance and systemic inflammation [28][127]. However, the factors that determine visceral or subcutaneous fat distribution remain unknown. Central obesity induces adipocyte hypertrophy and hyperplasia, macrophage infiltration, endothelial cell activation, and ectopic fat disposition due to excessive energy accumulation. Larger adipocytes are correlated with dysregulated adipokine expression, and hypertrophic adipocytes are prone to producing proinflammatory molecules [29][128]. In hypertrophic adipose tissue, local hypoxia can occur due to reduced blood flow relative to the size and number of adipocytes, which leads to reduced adiponectin production and increased proinflammatory cytokine expression [30][129]. Furthermore, obesity not only leads to increased macrophage infiltration in adipose tissue but also triggers their polarization as M1 macrophages producing proinflammatory cytokines and inducible nitric oxide synthase (iNOS) [31][130]. Through these processes, elevated cytokines and chemokines recruit monocytes that adhere to endothelial cells and elevate the expression of vascular adhesion molecules such as ICAM, VCAM, and E-selectin [32][131]. This ‘vicious cycle’ together with chronic inflammation in adipose tissue leads to various complications of metabolic syndrome such as hepatic fibroinflammatory injury, systemic arterial dysfunction, and insulin resistance. In this context, the adipokines and cytokines discussed above play pivotal roles in chronic inflammation, macrophage aggregation, and hypoxia and contribute to the variety of complications called metabolic syndrome (Figure 1).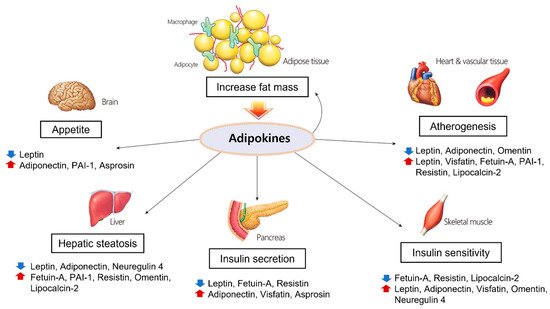
5. The Roles and Associated Mechanisms of Adipokines in Cardiovascular Diseases
5.1. Adiponectin
Adiponectin knockout mice exhibited a significantly increased expression of E-selectin, which is implicated in leukocyte rolling and leukocyte adhesion [36][135]. Aortic ring tissues derived from adiponectin knockout mice showed a decrease in endothelial NOS expression that might cause a defect in vasodilation. This was reversed by treatment with recombinant adiponectin [37][136]. In another study, adiponectin administration in obese rats increased endothelial NOS by activating the AMPK pathway and promoting NO production. This resulted in the relaxation of the aortic ring [38][137]. In cultured human umbilical vein endothelial cells, adiponectin showed a protective effect against angiotensin-II-induced vascular endothelial damage [39][138]. In addition, adiponectin attenuated angiotensin-II-induced NADPH oxidase activation in renal proximal tubular cells [40][139]. These studies suggest that adiponectin production is closely related to endothelial function in vasodilation.
5.2. Leptin
Leptin increased the vasodilatation of rat aortic rings in vitro via a nitric oxide (NO)-dependent mechanism [41][150], but another study showed that leptin had no effect on hemodynamics, even after blocking NO generation [42][151]. Moreover, leptin synthesis was found to increase when cultured with angiotensin II adipose cells and rats in vivo [43][152]. Leptin with adipose-tissue-derived angiotensin II can promote obesity-related hypertension [44][153]. In an in vitro study using endothelial cells of the human umbilical vein, leptin induced chronic oxidative stress in endothelial cells and promoted atherogenesis [45][154].
