Antimicrobial resistance (AMR) is a global health issue that plays a significant role in morbidity and mortality, especially in immunocompromised patients. It also becomes a serious threat to the successful treatment of many bacterial infections. The widespread and irrelevant use of antibiotics in hospitals and local clinics is the leading cause of AMR.
1. Introduction
COVID-19 infections have far exceeded bacterial co-infection and mortality rates compared to other common respiratory viral infections [1][2]. The co-infection of SARS-CoV-2 with other microbes, mainly bacteria and fungus, is a determining factor in COVID-19 development, making diagnosis, treatment, and prognosis more complicated. In individuals with COVID-19, bacterial co-infection has been linked to disease progression and prognosis. This scenario increases the need for critical care units, antibiotic therapy, and mortality [2][3]. Unfortunately, due to their widespread use, human may face the emergence of multi-drug resistant (MDR) pathogens leading to reduced efficacy of most potent antimicrobials [2][3][3,4]. AMR is a global problem that poses a severe threat to the success of treating a wide range of bacterial infections and affects many hospitalised patients, and most probably becomes a serious threat to the patients who are admitted to the SICUs [4][5][5,6].
Overall, the selection and development of highly drug-resistant bacteria due to the increased use of antibiotics and disinfectants may impact the clinical prognosis of severe COVID-19 patients receiving emergency hospital care, resulting in poor patient outcomes
[6][7][7,8]. In this context, highly and extensively drug-resistant organisms have been documented to cause significant co-infections in COVID-19 patients, and mortality has recently been recorded in situations when bacterial co-infections were reported in COVID-19 patients
[8][9][9,10].
The bacterial co-infections that arise during SARS-CoV-2 infection must be identified and characterised in a timely fashion
[10][11][11,12]. Several studies have looked into the prevalence of bacterial co-infections in COVID-19 patients, finding highly heterogeneous distributions (with differences of >50%) that can be attributed to clinical and epidemiological characteristics of each geographical location, as well as diagnostic methods and criteria, used
[12][13][14][13,14,15].
2. Antibiotic Susceptibility Patterns of Clinical Isolates
The antibiotic resistance patterns of individual bacterial isolates are shown in
Figure 1,
Figure 2,
Figure 3 and
Figure 4.
Figure 1. The antibiotic resistance patterns in (A) Staphylococcus aureus, (B) Streptococcus agalactiae, (C) Enterococcus faecalis. * Not reported in urinary isolates. ** Reported in urinary isolates only.
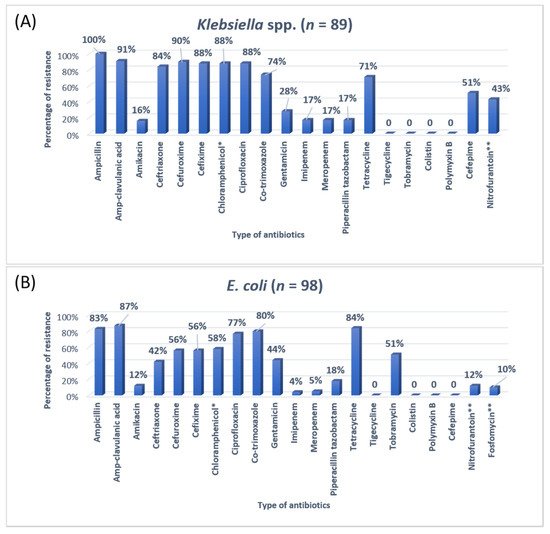
Figure 2. The antibiotic resistance patterns in (A) Klebsiella spp, (B) Escherichia coli * Not reported in urinary isolates. ** Only reported in urinary isolates.
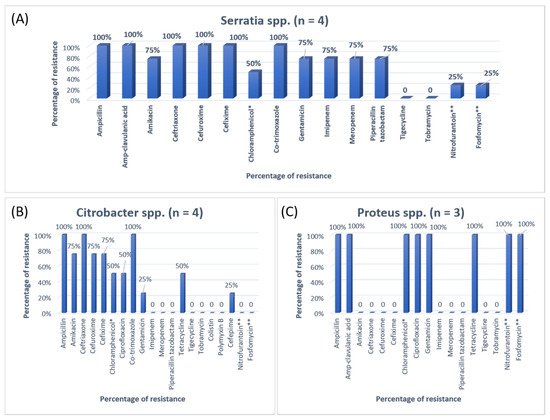
Figure 3. The antibiotic resistance patterns in (A) Serratia spp., (B) Citrobacter spp., (C) Proteus spp. * Not reported in urinary isolates. ** Only reported in urinary isolates.
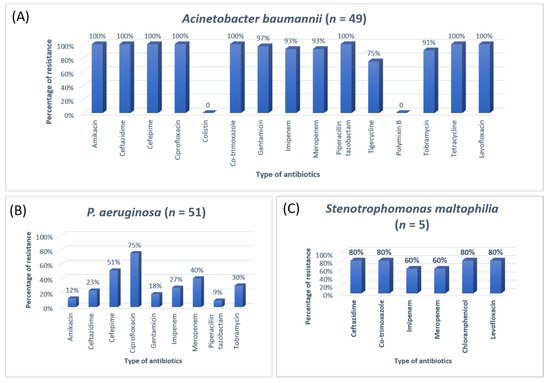
Figure 4. The antibiotic resistance patterns in (A) Acinetobacter baumannii, (B) Pseudomonas aeruginosa, (C) Stenotrophomonas maltophilia.
3. Research Findings
Antibiotics are the most commonly prescribed drugs among hospitalised patients, especially in SICUs. It is imperative to use the appropriate antibiotics in intensive care units with few prescriptions as an acceptable quality of care, infection control, cost reduction, and length of hospital stay [15][16][17][17,18,19]. Patients admitted in the SICU are critically ill requiring prescribed the medicine without waiting for the culture reports that give information about the antimicrobial resistance pattern of the suspected organism for a specific cause [18][19][20,21]. In terms of the culture reports, there is no possibility to wait for reports because these take a minimum of 48 h, and as a result antibiotic resistance occurs in patients admitted to the SICUs [20][22]. The current study was conducted among COVID-19 patients admitted in SICUs of tertiary care hospitals, requiring monitoring and special care to analyse the antibiotics utilisation pattern and determine the prevalence of AMR.
A previous study on microbial infection and antibiotic resistance patterns in COVID-19 patients admitted in SICUs of tertiary care hospitals showed that Pseudomonas was the most common organism identified in the medical ICU, followed by Klebsiella pneumonia [21][23]. A study on the prevalence of microorganisms and bacterial resistance in the SICU of the Bangabandhu Sheikh Mujib Medical University of Bangladesh showed that the maximum identified organism was Acetobacter. (45.4%), of P. aeruginosa (32.2%), Proteus (11%), Klebsiella Klebsiella pneumoniaepneumoniae 10%, and E. coli (3%) were identified [22][24]. A study by Mehta et al., 2015 on ICU patients revealed that the Pseudomonas spp. (29.1%) was the most common organism, followed by Acinetobacter spp. (27.5%) [16][18]. Another previous analysis of AST and bacteriology profile on patients at tertiary care hospitals in Ahmadabad showed that Acinetobacter spp. [30.9%] was the most common organism, after coming Klebsiella spp. (29.8%) and P. aeruginosa (22.9%) [17][19]. However, in the current study, the most common isolated organism was E. coli (38%), followed by Klebsiella pneumoniae (24%), P. aeruginosa (14%). While Streptococcus agalactiae, Citrobacter freundii, Serratia liqeuficiens, and Stenotrophomonas maltophilla were 1.7%, 1.1%, 1.1% and 1.4%, respectively.
In Jordan, a study was conducted to see the AMR rates, the prevalence of bacterial infections, and misuse of antibiotics. They conducted a study to resolve the resistance rate of Gram-negative bacteria in patients admitted to the ICU of Prince Hasen hospital. It revealed that
P. aeruginosa was the most resistant pathogen and broad-spectrum antibiotic-resistant bacteria
[14][15]. A similar previous study on the uropathogens of ICU showed that
E. coli was highly susceptible to imipenem, meropenem, and nitrofurantoin
[23][25]. The second most common organism in the current study was
Klebsiella pneumoniae, 100% resistant to ampicillin and 91% to Amp-clavulanic acid. For
Klebsiella pneumoniae, the treatment option was amikacin and gentamicin. Work on the AMR pattern of the bacterial pathogen in the ICU of Fatimawati hospital showed high resistance to cephalexin (96.3%), cefotaxime (64.3%), and ceftriaxone (61.0%) shown by
P. aeruginosa. The most effective antibiotic against
P. aeruginosa was amikacin after imipenem (81.3%) and meropenem (75.2%).
Klebsiella pneumoniae showed resistance to cephalexin, ceftriaxone, ceftazidime (86.6%) (75.9%) (73.4%) respectively
[24][26]. In the current study, the most common organism,
E. coli, showed ampicillin resistance (83%) and Amp-clavulanic acid (87%). In that case, the most effective antibiotic was imipenem and meropenem. Most
E. coli showed high sensitivity to the imipenem and meropenem (96%). Imipenem and meropenem are usually used against both GPIs and GNIs. However, a study conducted in India showed that
Klebsiella was more susceptible to imipenem and nitrofurantoin and showed more resistance to penicillin and gentamicin
[16][18].
Most of the Gram-positive organisms in the current study were resistant to penicillin and tetracycline. For the treatment of the Gram-positive bacteria, linezolid, gentamicin, and vancomycin were used. Most Gram-negative organisms were resistant to two or more alarming antibiotics and shortly caused high mortality and morbidity. This will also affect the management of Gram-negative bacteria. Around 17 different types of antibiotics were used in the ICU. Carbapenem, fluoroquinolones, aminoglycoside, and quinolones were highly consumed among all the antibiotics. In a study in India, the high consumption of beta-lactam, carbapenem, metronidazole was observed. Another study in Nepal showed that ampicillin, metronidazole, and amoxicillin were the most common antibiotics
[25][27]. Another research study showed that beta-lactam, nitroimidazoles, and fluoroquinolones were commonly used in an ICU
[11][12]. The prevalence of MRSA in the current study was 16%, VRE was 11%, and CRE 17%, showing a high rate of AMR against most effective drugs. These high rates of AMR in SICU patients are an alarming situation for all of the clinicians and researchers and the time to take some actions to ensure the correct usage of antibiotics.
Results of the current study showed that approximately 36% of COVID-19 patients had pneumonia followed by aspiration, diabetes mellitus (DM), chronic obstructive pulmonary disease (COPD), ischemic heart disease (IHD) and hypertension. UTIs, meningoencephalitis and sepsis followed by different comorbidities were also diagnosed. COVID-19 with bacterial pneumonia were the most important cause of distress and mortality. DM, HTN, CKD were significant comorbidities among ICU patients. Many patients had DM and HTN at the same time. Females experienced more complications and comorbidities than males. However, a previous systematic review and meta-analysis by Yang et al. (2020) reported that HTN and diabetes were the most common comorbidities, followed by cardiovascular diseases (CVD) and pneumonia. The pooled odds ratio of HTN, pneumonia and CVD in severe patients was lower than in non-severe patients
[26][28].
4. Conclusions
The current study points to a significant prevalence of bacterial infections and high AMR rates in COVID-19 patients, especially with specific comorbidities and complications, making it challenging to identify the priority of treatment groups and improve the care of these infections. These high rates of AMR may lead to high mortality rates; that is why a specific antibacterial usage policy of institutions and SICUs are needed to control this problem. Periodic audits are required to monitor compliance with these guidelines. In a hospital setup, antibiotic sensitivity and AMR patterns must be considered to direct the infection control expert or clinician to begin empirical antibiotics in severe cases. An intensive and systematic effort is needed to quickly classify high-risk patients and minimise the irrelevant use of antibiotics, one of the primary reasons for increased AMR rates in developing countries.