Transplacental gene delivery (TPGD) is a technique for delivering nucleic acids to fetal tissues via tail-vein injections in pregnant mice. After transplacental transport, administered nucleic acids enter fetal circulation and are distributed among fetal tissues. In 1995, TPGD was established by Tsukamoto et al., and its mechanisms, and potential applications have been further characterized since. In 2019, Nakamura et al. demonstrated that intravenous injection of plasmid DNA containing genome editing component (CRISPR/Cas9 system) produced indels in fetal myocardial cells. In the future, this unique technique will allow manipulation of fetal cell functions in basic studies of fetal gene therapy.
- gene delivery
- transplacental gene delivery (TPGD)
- TPGD for acquiring genome-edited fetuses
1. Definition
Transplacental gene delivery (TPGD) is a technique for delivering nucleic acids to fetal tissues via tail-vein injections in pregnant mice. After transplacental transport, administered nucleic acids enter fetal circulation and are distributed among fetal tissues.
2. Mechanism
The development of efficient methods for the transfer of nucleic acids to fetuses is an important goal for in utero gene therapy. Many of these approaches require ex vivo handling procedures that temporally expose fetuses, and micropipette injections of nucleic acids under anesthetic conditions are time-consuming and labor-intensive. As an alternative administration route, transplacental transfer of plasmid DNA constructs was reported after tail-vein injection into dam. After transplacental transport, administered nucleic acids reach the fetal circulation and efficiently transfect fetal cells. This approach represents a noninvasive and convenient method for the transfection of fetal tissues, and is hereafter referred to as transplacental gene delivery (TPGD). Since the work of Tsukamoto et al.[1], supporting data have been generated, and TPGD has been achieved with nucleic acids derived from plasmids or viral vectors[2]. Recently, Nakamura et al. elicited CRISPR/Cas9-mediated mutations in a target locus of embryonic cells for the first time using TPGD for acquiring genome-edited fetuses (TPGD-GEF)[3].
During TPGD, the placenta plays an important role in the transport of nucleic acids from the blood stream of pregnant dams to post-implantation embryos. The placenta is a highly specialized tissue that contributes to fetal developing by controlling the flow of nutrients through the umbilical cord to the uterine wall, by eliminating metabolic decomposition products and by mediating exchange gas between maternal and fetal circulatory systems[4].
Kikuchi et al. speculated about the mechanisms underlying TPGD[5], as shown schematically in Figure 1. They suggest that on E5.5 to E9.5, plasmid DNA is introduced with poor efficiency because the placenta is immature. At this time, nutrients, including lipids, are known to be taken up by the visceral endoderm (VE) or yolk sac, and are then transported to embryos by diffusion or vitelline circulation[6][7]. DNA/lipid complexes in maternal blood may also be transferred to embryos via the VE or yolk sac (arrowheads in Figure 1). However, under these conditions, most DNA/lipid complexes may be trapped in the VE, and small amounts may be taken up and transported to the embryo by vitelline circulation. In contrast, transfer of nutrients commences at the placenta during E10.5 to E13.5. As shown by arrowheads in area A of Figure 1, some plasmid DNA/lipid complexes may be transferred to the umbilical vein at the placenta and then into embryonic circulation. Yet some plasmid DNA/lipid complexes may also enter the blood vessels of the decidua (arrowheads in area B in Figure 1). Tail-vein injections of trypan blue dye demonstrated that some exogenous DNA can be transferred to the embryo via the vitelline veins, but most is trapped at the yolk sac, especially at the portion that is proximal to the placenta. Hence, establishment of placental circulation may account for increased transfer of plasmid DNA/lipid complexes on E12.5 and thereafter. Kikuchi et al. also suggest that yolk sac circulation may function as a route for the transfer of DNA/lipid complexes from maternal circulation to the fetus[5].
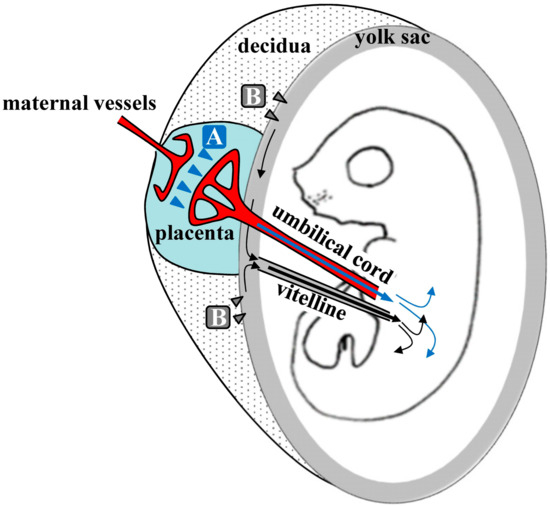
Figure 1. Hypothetical mechanism of transplacental gene delivery (TPGD) as suggested by Kikuchi et al.[5]; Following TPGD on E12.5, when placental circulation is established, intravenously injected plasmid DNA/lipid complexes may be transferred from maternal blood to the fetus via at least two routes. Flow via the placenta to the embryo is indicated by the blue arrowheads (area A); injected plasmid DNA is transferred beyond the blood-placenta barrier (BPB) and enters the umbilical cord. Flow from the decidua to the yolk sac is indicated by the gray arrowheads (area B); some DNA becomes trapped in yolk sac and is transferred to the embryo after the establishment of functional placental circulation.
Because the placenta is the most species-specific organ, hypotheses that are generated from rodent experiments extrapolate poorly to humans [8]. However, accumulating evidence suggests that nanoparticles in the maternal blood stream can be transferred to the fetus via the placenta. Transfer of nanoparticles through the placental barrier requires passage through syncytiotrophoblast (ST) and villous stroma (VS) layers, and through endothelial cells of fetal capillaries, evoking paracellular and transcellular pathways, respectively [9]. Nanoparticles of less than 25 nm in diameter can pass through the paracellular pathway and penetrate ST layers via passive diffusion, thus entering the VS layers with ease. Cationic nanoparticles are also increasingly considered as vehicles for gene delivery and may directly fuse to ST layers, because basal ST membranes are negatively charged. Other nanoparticles usually employ the transcellular pathway, as exemplified by endocytosis and exocytosis. Alternatively, nanoparticles may be taken up by STs via phagocytosis, clathrin-mediated endocytosis, caveolae-mediated endocytosis, or micropinocytosis. However, these routes are subject to endosomal escape pathways, lysosomal secretion, and multivesicular body (MVBs)-related secretions. In all cases, nanoparticles diffuse into fetal circulation through endothelial cells of fetal capillaries (Figure 2).
In human placenta tissues, most drugs of less than 600 Da are known to reach the fetus by passive diffusion, whereas drug molecules of over 1000 Da[10] require transport via specific receptor-mediated pathways on ST surfaces. Macromolecules, such as immunoglobulin G antibody (~150 kDa) and vitamin B12 (1.3 kDa), are known to cross the placenta[11]. Hence, such receptor-mediated transfer systems may be critical for delivery of large molecules using TPGD in humans. The mechanisms underlining transplacental transport of substances remain poorly understood, however, and further research will be needed prior to application of TPGD in humans.
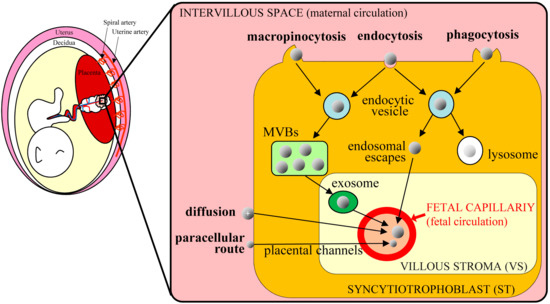
Figure 2. Schematic of nanoparticle transplacental transport mechanisms in humans (based on Zhang et al. [9]); nanoparticles in the maternal circulation cross the placental barrier and are transported to the fetus via various routes. The transcellular route is mediated by endocytosis and exocytosis. Nanoparticles are taken up via macropinocytosis, endocytosis, and phagocytosis in syncytiotrophoblast (ST) cells and are then exocytosed from endocytic vesicles through the multivesicular bodies (MVBs)-related secretion and endosomal escape. After entering the villous stroma (VS), nanoparticles cross endothelial cells of fetal capillaries by diffusion or via exosomes and enter the fetal blood. Some cationic nanoparticles can move toward fetal capillaries by simple diffusion, reflecting electrostatic interactions with cell membranes. Very small nanoparticles can pass ST cells through placental channels and enter the VS through the paracellular route.
In future, TPGD may be possible to manipulate, (i) fetal genomes of domestic animals, which are more difficult to manipulate than fetuses from small animals such a mice, and (ii) genomes of fetuses from animals for which in vitro cultivation systems are not fully established. In addition, it seems that TPGD and TPGD-GEF are still in the process of improvement but have a great potential as future fetal gene therapies.
This publication can be found here:https://www.mdpi.com/1422-0067/20/23/5926
References
- Makoto Tsukamoto; Takahiro Ochiya; Sho Yoshida; Takashi Sugimura; Masaaki Terada; Gene transfer and expression in progeny after intravenous DNA injection into pregnant mice. Nature Genetics 1995, 9, 243-248, 10.1038/ng0395-243.
- Shingo Nakamura; Satoshi Watanabe; Naoko Ando; Masayuki Ishihara; Masahiro Sato; Transplacental Gene Delivery (TPGD) as a Noninvasive Tool for Fetal Gene Manipulation in Mice. International Journal of Molecular Sciences 2019, 20, 5926, 10.3390/ijms20235926.
- Shingo Nakamura; Masayuki Ishihara; Naoko Ando; Satoshi Watanabe; Takayuki Sakurai; Masahiro Sato; Transplacental delivery of genome editing components causes mutations in embryonic cardiomyocytes of mid-gestational murine fetuses. IUBMB Life 2019, 71, 835-844, 10.1002/iub.2004.
- Neil M. Gude; Claire T. Roberts; Bill Kalionis; Roger G. King; Growth and function of the normal human placenta. Thrombosis Research 2004, 114, 397-407, 10.1016/j.thromres.2004.06.038.
- Natsuko Kikuchi; Shingo Nakamura; Masato Ohtsuka; Minoru Kimura; Masahiro Sato; Possible mechanism of gene transfer into early to mid-gestational mouse fetuses by tail vein injection. Gene Therapy 2002, 9, 1529-1541, 10.1038/sj.gt.3301818.
- David A. Beckman; John B Lloyd; Robert L Brent; Investigations into mechanisms of amino acid supply to the rat embryo using whole-embryo culture.. The International Journal of Developmental Biology 1997, 41, 315-318, DOI.
- Yuko Terasawa; Sylvaine J. Cases; Jinny S. Wong; Haris Jamil; Sumana Jothi; Maret G Traber; Lester Packer; David A Gordon; Robert L Hamilton; Robert V Farese; et al. Apolipoprotein B-related gene expression and ultrastructural characteristics of lipoprotein secretion in mouse yolk sac during embryonic development. Journal of Lipid Research 1999, 40, 1967–1977, DOI.
- Katerina Guschanski; Maria Warnefors; Henrik Kaessmann; The evolution of duplicate gene expression in mammalian organs. Genes & Development 2017, 27, 1461-1474, 10.1101/gr.215566.116.
- Baozhen Zhang; Ruijing Liang; Mingbin Zheng; Lintao Cai; Xiujun Fan; Surface-Functionalized Nanoparticles as Efficient Tools in Targeted Therapy of Pregnancy Complications.. International Journal of Molecular Sciences 2019, 20, 3642, 10.3390/ijms20153642.
- Anupa R. Menjoge; Amber L. Rinderknecht; Raghavendra S. Navath; Masoud Faridnia; Chong J. Kim; Roberto Romero; Richard K. Miller; Rangaramanujam M. Kannan; Transfer of PAMAM dendrimers across human placenta: prospects of its use as drug carrier during pregnancy.. Journal of Controlled Release 2010, 150, 326-38, 10.1016/j.jconrel.2010.11.023.
- Henning Schneider; Richard K. Miller; Receptor-mediated uptake and transport of macromolecules in the human placenta. The International Journal of Developmental Biology 2010, 54, 367-375, 10.1387/ijdb.082773hs.
