Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Beatrix Zheng and Version 1 by Luis Alberto Medina.
Spinal cord injury (SCI) refers to the damage suffered in the spinal cord by any trauma or pathology. This work determined whether 99mTc-GA-5, a radiotracer targeting Glial Fibrillary Acidic Protein (GFAP), can reveal in vivo the reactivation of astrocytes in a murine model with SCI.
- spinal cord injury
- GFAP
- GA-5 monoclonal antibody
- 99mTc-GA-5
- microSPECT/CT imaging
- astrogliosis
- molecular imaging
1. Introduction
Traumatic spinal cord injury (SCI) is a public health problem that affects the economically active population, leading to neurological, sensory, and motor deficits [1,2,3,4][1][2][3][4]. The World Health Organization (WHO) estimates worldwide incidence varies between 40 and 80 cases per million habitants [1]. Long-term neurological recovery continues to be limited, producing physical, emotional, and economic consequences for patients, families, and society. To date, effective therapies for SCI remain challenging, which has encouraged an exhaustive search focused on the characterization of the pathophysiology of this condition and the development of innovative therapeutic methods that aim to repair the injured spinal cord [5,6][5][6].
The Glial Fibrillary Acidic Protein (GFAP) is the main structural protein of the filaments within the cytoskeleton of astrocytes and acts as a marker of mature astrocytes. The roles attributed to GFAP in the Central Nervous System (CNS) include the suppression of neuronal proliferation and neurite extension in the mature brain, formation of a physical barrier to isolate damaged tissue, participation in cerebellar motor learning, blood flow regulation following ischemia, participation in the blood-brain barrier, supporting myelination, and providing mechanical strength [7]. Recent evidence suggests that GFAP and its breakdown products are rapidly released into biofluids after traumatic brain and spinal cord injuries and stroke, making them robust candidate biomarkers for such disorders [8]. Consequently, GFAP biomarkers can be further developed into theranostic molecules that can help to treat and diagnose CNS injury.
Astrocytes are the most abundant cell type in the mammalian CNS [9]. After SCI, astrocytes become reactive at the site of injury and beyond, a process known as astrogliosis, characterized by profound morphological, molecular, and functional changes in astrocytes. Astrogliosis can be demonstrated by immunohistochemistry using primary antibodies to GFAP [9,10,11][9][10][11]. Reactive astrocytes are an essential part of the multiple cellular and extracellular components of the glial scar that forms due to the cascade of inflammatory and pathological processes triggered by the initial mechanical injury to the spinal cord [12,13,14][12][13][14].
Molecular imaging technologies such as SPECT, PET, MRI, and optical imaging (i.e., bioluminescence imaging), make it possible to detect in vivo cellular and molecular aspects of specific targets in a spatial-temporal way [15]. In contrast to conventional diagnostic images, molecular imaging represents an emerging field that integrates molecular biology, chemistry, and radiology, giving an effective way to monitor therapeutic strategies, as well as to study physiological and pathological processes at the molecular level of multiple fields, including of course SCI research [16,17,18,19][16][17][18][19].
Considering the lack of molecular imaging studies to characterize the reactive astrocytic response in the rodent spinal cord after SCI, the present study was designed to test the hypothesis that the use of a radiolabeled antibody targeting GFAP after SCI will allow obtaining molecular images that translate the occurrence of astrogliosis due to the injury. Here, we report the procedures for the technetium-99m (99mTc) radiolabeling of the mouse anti-GFAP monoclonal antibody ((GA-5): sc-58766). The GA-5 reacts with the GFAP, and it is commonly used in immunohistochemical studies to identify astrocytes and other glial cells. Here, SPECT/CT molecular imaging procedures were implemented to evaluate the 99mTc-GA-5 (99mTc-anti-GFAP) as an in vivo radiotracer in a rat SCI model.
2. Insightful Analysis
Here, a molecular imaging protocol served as a platform to test the capacity of 99mTc-GA-5 (a radiolabeled anti-GFAP) to identify in vivo the astrocytic response in the spinal cord of rats subjected to SCI with a model that reproduces human SCI [20,21][20][21].
To accomplish this goal, before performing the imaging studies, we made sure that the radiolabeling efficiency and the radiotracer integrity met the required standards. We also confirmed, by radioimmunohistochemistry, that the RIC is bound to the cells and tissues of interest in a post-injury time-dependent manner. After solving these basic precepts, we designed the imaging protocol grounded on SPECT utilizing a dedicated small animal system (Albira small animal microPET/SPECT/CT imaging system). As a global result, our radiotracer selectively accumulates in glial cells (targeting primarily reactive astrocytes) as an active agent, facilitating the in vivo monitoring of astrogliosis.
GA-5′s receptors can be over or underexpressed in cells and tissues under physiological or pathological conditions and used as molecular targets [15]. The specific interaction between the receptor and its ligand (GFAP and 99mTc-anti-GFAP in our study) proved to be an effective strategy to evaluate this radiopharmaceutical’s amount and residence time in target tissues. By considering the temporal/spatial expression of GFAP in the current radioimmunohistochemistry assay (see Figure 31) and previous histological studies [22], we chose to perform our imaging study at 20 days after injury, as this is the timeframe where reactive astrocytes overexpress GFAP in the context of the glial scar formation.
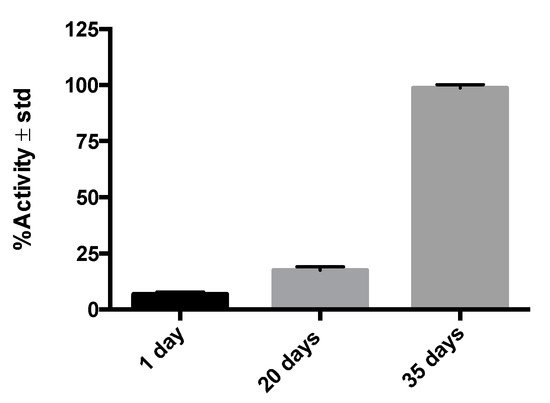

Figure 1. %Activity in post-injury histological samples (n = 3–6) on different days, illustrating the affinity of 99mTc-GA-5 for its receptors in cells of murine medullary tissue.
Contrary to most in vivo molecular imaging studies where the radioactive biomarker is administered intravenously, we used the intrathecal route successfully. When macromolecules, such as proteins, are administered intravenously, the blood-brain barrier limits their access to the CNS. However, when delivered intrathecally, macromolecules circumvent this barrier and effectively reach their target within the CNS [23,24,25][23][24][25]. The imaging strategy tested here was effective in revealing the cellular and molecular processes sought. The biomarker appears to be highly sensitive to GFAP expression changes and the distribution of the reactive astrocytes at and around the injury site.
SCI is a pathology with high physical, social, and economic repercussions [1,2,3,26,27,28,29][1][2][3][26][27][28][29] commonly occurring in vehicular accidents, sports-related injuries, or violent episodes, for which no therapies to restore neurologic deficit exist effectively. After an injury occurs, death of neurons and glial cells, ischemia, and inflammation, which is followed by the formation of a glial scar and cystic cavities in the spinal cord, are observed [30]. Due to the profound impact of astrogliosis on the progression of SCI, a better understanding of the cellular and molecular events in glial scarring is mandatory [31,32][31][32]. To date, the role of astrogliosis has been oversimplified in binary terms as good or bad [11,14][11][14]. It is considered beneficial because it aids in repairing the initial damage, stabilizes the spread of injury, and fosters axonal regeneration and functional recovery, but detrimental because it provides both a physical and chemical barrier to regenerating axons [7,13,16,31,32][7][13][16][31][32].
There is no diagnostic strategy for in vivo selective identification of GFAP present in reactive astrocytes through molecular imaging methods. Here, we demonstrate that the rapid identification of astrogliosis through molecular imaging in a murine SCI model is feasible without characterizing the mechanisms that regulate astrocyte reactivity and scar formation. We consider that the main contribution of this study lies in the development of a tool that will be useful to understand better and monitor astrogliosis, a controversial topic of the most significant relevance in the pathophysiology of SCI.
Nuclear imaging (SPECT and PET scans) is widely used in cardiology, neurology, and oncology. Although no studies have been reported evaluating molecular imaging technologies targeting GFAP in SCI, some recent studies have reported the utility of nuclear imaging to reveal in vivo biological activity of events associated with SCI, such as acute inflammation in a rodent model [19].
References
- OMS Lesiones Medulares. Available online: http://www.who.int/mediacentre/factsheets/fs384/es/ (accessed on 25 February 2018).
- NINDS Spinal Cord Injury Information Page. Available online: https://www.ninds.nih.gov/Disorders/All-Disorders/Spinal-Cord-Injury-Information-Page#disorders-r1 (accessed on 28 August 2017).
- Ruiz, A.D.; Sahagún, G.G.; Castañeda, C.R. Estrategias neuroprotectoras después de una lesión traumática de la médula espinal. Rev. Med. IMSS 2002, 40, 437–455.
- NSCISC National Spinal Cord Injury Statistical Center. Available online: https://www.nscisc.uab.edu/ (accessed on 24 February 2018).
- Hernández, J.; Torres-Espín, A.N. Adult stem cell transplants for spinal cord injury repair: Current state in preclinical research. Curr. Stem Cell Res. Ther. 2011, 6, 273–287.
- Cizkova, D.; Murgoci, A.N.; Cubinkova, V.; Humenik, F.; Mojzisova, Z.; Maloveska, M.; Cizek, M.; Fournier, I.; Salzet, M. Spinal cord injury: Animal models, imaging tools and the treatment strategies. Neurochem. Res. 2020, 45, 134–143.
- Brenner, M. Role of GFAP in CNS injuries. Neurosci. Lett. 2014, 565, 7–13.
- Yang, Z.; Wang, K.K.W. Glial fibrillary acidic protein: From intermediate filament assembly and gliosis to neurobiomarker. Trends Neurosci. 2015, 38, 364–374.
- Magaki, S.D.; Williams, C.K.; Vinters, H.V. Glial function (and dysfunction) in the normal & ischemic brain. Neuropharmacology 2018, 134, 218–225.
- Pekny, M.; Pekna, M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J. Pathol. 2004, 204, 428–437.
- Karimi-Abdolrezaee, S.; Billakanti, R. Reactive astrogliosis after spinal cord injury-beneficial and detrimental effects. Mol. Neurobiol. 2012, 46, 251–264.
- Yuan, Y.-M.; He, C. The glial scar in spinal cord injury and repair. Neurosci. Bull. 2013, 29, 421–435.
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte reactivity and astrogliosis after spinal cord injury. Neurosci. Res. 2018, 126, 39–43.
- Bradbury, E.J.; Burnside, E.R. Moving beyond the glial scar for spinal cord repair. Nat. Commun. 2019, 10, 3879.
- Reubi, J.C.; Maecke, H.R. Peptide-based probes for cancer imaging. J. Nucl. Med. 2008, 49, 17351738.
- Lo, W.C.; Hsu, C.H.; Wu, A.T.; Yang, L.Y.; Chen, W.H.; Chiu, W.T.; Lai, W.F.; Wu, C.H.; Gelovani, J.G.; Deng, W.P. A novel cell-based therapy for contusion spinal cord injury using GDNF-delivering NIH3T3 cells with dual reporter genes monitored by molecular imaging. J. Nucl. Med. 2008, 49, 1512–1519.
- Song, F.; Tian, M.; Zhang, H. Molecular imaging in stem cell therapy for spinal cord injury. Biomed. Res. Int. 2014, 2014, 759514.
- LeRoux, L.G.; Bredow, S.; Grosshans, D.; Schellingerhout, D. Molecular imaging detects impairment in the retrograde axonal transport mechanism after radiation-induced spinal cord injury. Mol. Imaging Biol. 2014, 16, 204–510.
- Albadawi, H.; Chen, J.W.; Oklu, R.; Wu, Y.; Wojtkiewicz, G.; Pulli, B.; Milner, J.D.; Cambria, R.P.; Watkins, M.T. Spinal cord inflammation: Molecular Imaging after Thoracic Aortic Ischemia reperfusion injury. Radiology 2017, 282, 202–211.
- Kjell, J.; Olson, L. Rat models of spinal cord injury: From pathology to potential therapies. Dis. Model. Mech. 2016, 9, 1125–1137.
- Minakov, A.N.; Chernov, A.S.; Asutin, D.S.; Konovalov, N.A.; Telegin, G.B. Experimental models of spinal cord injury in laboratory rats. Acta Nat. 2018, 10, 4–10.
- Baldwin, S.A.; Broderick, R.; Blades, D.A.; Scheff, S.W. Alterations in temporal/spatial distribution of GFAP- and vimentin-positive astrocytes after spinal cord contusion with the New York University Spinal cord injury device. J. Neurotrauma 1998, 15, 1015–1026.
- Nestrasil, I.; Shapiro, E.; Svatkova, A.; Dickson, P.; Chen, A.; Wakumoto, A.; Ahmed, A.; Stehel, E.; McNeil, S.; Gravance, C.; et al. Intrathecal enzyme replacement therapy reverses cognitive decline in mucopolysaccharidosis type I. Am. J. Med. Genet. Part A 2017, 173, 780–783.
- Ineichen, B.V.; Schnell, L.; Gullo, M.; Kaiser, J.; Schneider, M.P.; Mosberger, A.C.; Good, N.; Linnebank, M.; Schwab, M.E. Direct, long-term intrathecal application of therapeutics to the rodent CNS. Nat. Protoc. 2017, 12, 104–131.
- Wahl, A.-S.; Correa, D.; Imobersteg, S.; Maurer, M.A.; Kaiser, J.; Augath, M.A.; Schwab, M.E. Targeting Therapeutic antibodies to the CNS: A comparative study of intrathecal, intravenous, and subcutaneous Anti-Nogo a antibody treatment after stroke in rats. Neurotherapeutics 2020, 17, 1153–1159.
- Harkey, H.L., III; White, E.A., IV; Tibbs, R.E., Jr.; Haines, D.E. A clinician’s view of spinal cord injury. Anat. Rec. B New Anat. 2003, 271, 41–48.
- Pérez, R.; Martín, S.; Renán, S.; Ortiz, S.D. Aspectos epidemiológicos de la lesión medular de la población del Centro Nacional de Rehabilitación. Rev. Mex. Med. Física Rehabil. 2008, 20, 74–82.
- De Esclarín Ruz, A. Lesión Medular, Enfoque Multidisciplinario, 1st ed.; Panamericana, E.M., Ed.; Editorial Médica Panamericana S.A.: Madrid, Spain, 2011; ISBN 9788491106326.
- Rodríguez Fernández, M. Lesión Medular: Atención Sociosanitaria; Formación Alcalá: Alcalá la Real, Spain, 2020; ISBN 978-84-85539-77-2.
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 1–21.
- Tran, A.P.; Warren, P.M.; Silver, J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 2018, 98, 881–917.
- Orr, M.B.; Gensel, J.C. Spinal cord injury scarring and inflammation: Therapies targeting glial and inflammatory responses. Neurotherapeutics 2018, 15, 541–553.
More
