Liver resection is recognized worldwide as a potentially curative treatment for patients with primary and secondary malignancies and resectable disease. Preoperative 3D reconstructions and printing as well as augmented reality can increase the knowledge of the specific anatomy of the case and therefore plan the surgery accordingly and tailor the procedure on the patient. Furthermore, the indocyanine green retention dye is an increasingly used tool that can nowadays improve the precision during laparoscopic hepatectomies, especially when considering anatomical resection. The use of preoperative modern imaging and intraoperative indocyanine green dye are key to successfully perform complex hepatectomies such as laparoscopic parenchymal sparing liver resections.
1. Introduction
Liver resection is recognized worldwide as a potentially curative treatment for patients with primary and secondary malignancies and resectable disease
[1][2][1,2]. Depending on the histology, the tumor biology and the extent of the disease, different types of surgical procedures can be performed, combined or not with systemic chemotherapy. Metastases from colorectal cancer (CRLM) are the most frequent secondary liver disease and most commonly require a combination of chemotherapy and partial hepatectomies to spare as much liver parenchyma as possible and allow for subsequent surgery in case of recurrence. Hepatocellular carcinoma (HCC) is the most common primary liver tumor: patients generally present with underlying liver disease from chronic conditions (viral, alcoholic, metabolic…) and when scheduled for surgery, these require special considerations in the preoperative management to reduce the chance of morbidity and mortality
[3][4][3,4]. Given the ongoing debate in the literature, patients with HCC both undergo anatomical or non-anatomical liver resections depending on centers and geographic areas of treatment, with different preoperative and surgical implications
[5]. On the contrary, cholangiocarcinomas both intra- and extrahepatic, generally require liver resections associated with lymphadenectomy as these tumors has been associated with a higher chance of spreading to regional lymph node stations
[6][7][6,7].
Despite the initial skepticism, laparoscopic liver resections (LLRs) has gained widespread popularity among hepatobiliary surgeons and is nowadays performed for both standard and more complex hepatectomies
[8][9][10][11][12][13][14][15][13,14,15,16,17,18,19,20]. Given the lack of tactile sensation, the challenges with manipulations of the liver and the technical difficulties of performing intraoperative laparoscopic ultrasound, preoperative planning and intraoperative guidance is even more important in laparoscopy than in open surgery to safely carry out complex and oncologically safe hepatectomies.
2. Preoperative Imaging
A safe and successful liver resection should be oncologically radical, respect the anatomy, and leave as much healthy parenchyma as possible with adequate inflow, outflow, and bile drainage. These principles are important to reduce the possibility of tumor recurrence on one hand, and decrease the chance of post hepatectomy liver failure (PHLF) on the other hand
[16][21]. Because this balance in liver surgery is very important, the definition of the anatomy of the organ including major vascular structures and bile ducts and the relationship of these structures with the disease, requires dedicated and precise studies. With recent advancements in technology, routine imaging such as CT scans, MRIs and PET/CTs have been coupled with more advanced imaging techniques that serve as a better tool to preoperatively study the anatomy of the patient and the extent of the disease to eventually plan the surgery accordingly. Indeed, the 2D pictures obtained by standard diagnostics can be further processed to obtain 3-dimensional imaging that can further increase the knowledge of the anatomy and more accurately depict the vascular and biliary structures
[17][22]. This allows the surgeon to tailor the procedure on the specific disease and anatomy, and therefore on the patient. Indeed, 3D modeling contributes to increase the precision of liver surgery by allowing the estimation of the total liver volume and of the single portal territories, the identification of the lesions and the relationship with the major structures and the delineation of resection lines along liver segments, eventually allowing for a preoperative simulation of the planned resection (
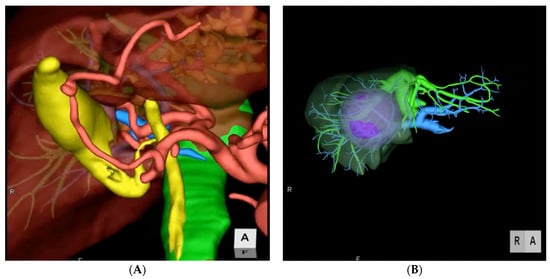
Figure 1). Many different medical softwares are available on the market to render 3D images from conventional 2D CTs or MRIs (Syngo.via Liver Analysis, Slicer, Zionstation, Osirix, MeVis…). All of these produce a detailed reconstruction that can be used to plan many different types of procedures, from wedges and anatomical segmentectomies to major hepatectomies with or without bile duct and vascular reconstructions. In hilar cholangiocarcinoma for example, 3D imaging allows to spatially recognize the exact location of the tumor and the extent of resection, together with the exact point of transection and reconstruction of the biliary continuity to have a oncologically free margin, anticipating the type of surgery
[18][23]. Operations performed with 3D modeling showed significantly shorter operative time, lower blood loss and fewer margin positivity
[19][24].
Figure 1. Two examples of preoperative 3D imaging. (A) Reconstruction of arterial, portal, and venous system and biliary tree. (B) Reconstruction of vascular structures, portal territories and tumor.
All the above-mentioned technologies can be taken in the operative room and used to guide the resection and to correct the task depending on the intraoperative findings. Indeed, a screen during laparoscopic liver resections can be dedicated to the 3D reconstructions to constantly review the images. Keeping track of every single step allows to roadmap the procedure, decreasing unexpected events and minimizing futile maneuvers. Furthermore, the latest hologram technology can eliminate the need for dedicated screens in the operative room, allowing the direct interaction of the surgeon with the model thanks to the Microsoft HoloLens technology, using glasses and enhancing virtual reality
[20][21][28,29].
3. Indocyanine Green Dye Fluorescence
Indocyanine green (ICG) is a sterile, anionic, water-soluble but relatively hydrophobic, tricarbocyanine molecule with a molecular mass of 776 Daltons
[22][30]. Following intravenous injection, ICG is rapidly bound to plasma proteins and rapidly extracted by the liver without modifications and nearly exclusively excreted by the liver appearing unconjugated in the bile about 8 min after injection, depending on liver vascularization and function
[23][24][31,32] ICG becomes fluorescent once excited using near-infra-red (NIR) light and the fluorescence released by ICG can be detected using specifically designated scopes and camera. Different surgical specialties have implemented the use of ICG in clinical practice. Indeed, fluorescence imaging can be of great help in the intraoperative visualization of anatomical structures such as lymphatics and lymph nodes, vessels, parenchyma such as the kidney and hollow viscus’ vascularization. Therefore, it is now widely accepted as an innovative and useful tool in many different surgical specialties such as ophthalmology, neurosurgery, pediatric, colorectal, gastric, and esophageal surgery
[23][25][26][27][31,33,34,35].
Given its properties, ICG has numerous applications in liver surgery. First and foremost, the biliary secretion of the dye allows to visualize the bile ducts during cholecystectomies, donor hepatectomies or liver resections with bile duct resections, allowing to precisely identify the structures that need to be saved or cut. In a recent consensus conference on the applications of ICG in hepatobiliary surgery, experts found that the use of this technology, significantly improved the accuracy of bile duct identification and reduced the incidence of iatrogenic injuries
[28][36]. Furthermore, ICG can also be used to detect bile leaks from the cutting surface, although there are only preliminary reports on the usefulness of this technology in this setting
[29][37].
Administered 1–2 weeks before the surgery, ICG nicely helps to intraoperatively identify the tumor, either HCC, cholangiocarcinoma or CRLM. The dosage and timing of administration of the dye varies according to the disease and type of parenchyma: the general principle is that for normal liver parenchyma, a dose of 0.3–0.5 mg/Kg is administered 10 days before surgery. In cirrhotic or fibrotic livers, the dose should be decreased given the impaired washout properties of the parenchyma and should therefore not exceed 0.3 mg/Kg. Also, the timing of administration can be modified increasing or decreasing the dose of ICG whenever the surgery is earlier or later that 10 days from the infusion respectively. The dye is accumulated and then progressively washed out by the normal liver parenchyma, while it is retained by the tumor, given the altered anatomy causing distortion of the peripheral bile structures. Ishizawa et al. previously described that tumor fluorescence also varies depending on histological differentiation of tumors, with different patterns and accumulation timing
[23][24][31,32].
ICG can also be administered intraoperatively to identify the portal territories and therefore perform anatomical liver resections
[30][38]. Glissonian pedicles of first, second or third order, can be isolated either from the liver hilum or from the parenchyma. Then, a positive or negative staining technique is used. The positive staining technique reproduces what has been previously described by Makuuchi et al. for systematic segmentectomies
[31][39]. The portal pedicle of the territory that needs to be resected is identified by ultrasonography and directly punctured and injected with ICG. The segment that needs to be resected will now shine green and the remaining liver will appear dark at near-infrared light. This technique is challenging in laparoscopy as requires advanced skills with intraoperative ultrasound and confidence with needle handling and targeting either trans- or intraabdominally. The negative staining technique is more frequently performed in laparoscopy as it is easier and ensures the same clear vision demarcation of the portal territories. The Glissonian pedicle is isolated from the hepatic hilum using the Glissonian approach, or transparenchymally using the transfissural approach. Glissonian pedicles of lobes, sections, segments and subsegments can be encircled from the liver hilum using the extra-fascial approach and respecting the Laennac’s capsule as described by Sugioka et al
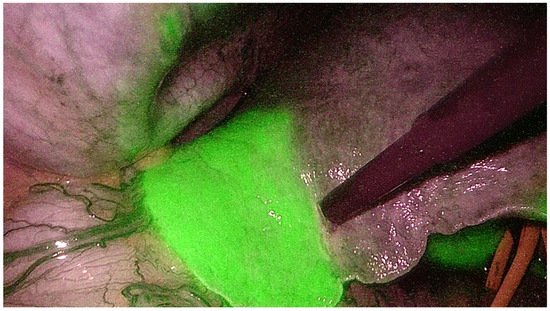
[32][40]. Once the pedicle is identified, it is temporarily clamped with an endoscopic bulldog and the ICG is administered intravenously. With this technique, the whole liver will shine green while the portal territory that needs to be resected will appear dark using near-infrared light camera (
Figure 2)
[33][41]. Both techniques are gaining worldwide popularity as the ICG allows to achieve a perfect anatomical resection. Indeed, it guarantees the identification of the intersegmental planes and the borders of the resection not only on the surface but also in the deep liver, removing the whole tumor-bearing area and leaving a well-vascularized parenchyma. In the era of minimally invasive liver surgery, ICG fluorescence helps identify liver segments boundaries and localize tumors, even in cirrhotic livers
[34][42]. This technology, may help overcome the limitations of LLR (lack of tactile sensation, challenges in ultrasound) for both anatomical and non-anatomical resections. Intraoperative ICG guidance during laparoscopic hepatectomies has some important limitations. First, specific technology is needed, with dedicated screens and laparoscopic equipment, and despite it is nowadays widely available, it still represents an extra cost. Dosage and timing are very important, and a wrong administration of the dye will make the technology useless as all the liver will shine green or nothing will shine at all. Few cases of hypersensitivity to the dye have been reported but thorough investigation of the past medical history should anyhow be performed. Finally, both negative and positive staining are challenging and require specific technical skills which need to be acquired with dedication and significant learning curve.
Figure 2.
Indocyanine green dye negative staining technique to identify the portal territories during anatomical hepatectomy.
4. Laparoscopic Anatomical Parenchymal Sparing Liver Resections
The above-mentioned technological advancements, allowed to push the limits in liver surgery and perform technically major surgeries that were once considered not feasible. These tools are of great help during anatomical liver resections. Anatomical resections include the removal of an area that is defined by the vascular supply of a portal pedicle (either main, sectorial, segmental or subsegmental). Anatomical resections allow to remove the tumor bearing area and this has been shown in retrospective reports to improve the oncological outcomes and to reduce local recurrence compared to non-anatomical resections in patients with HCC
[35][36][43,44]. Moreover, anatomical resections allow to reduce the remnant liver ischemia that is common issue after partial resection, and this has been associated with worse long-term outcomes for both HCC and CRLM
[37][38][45,46].
Anatomical studies show that the Glissonian pedicles first bifurcate into right and left hemi-livers. Then, secondary bifurcation divides the right hemi-liver into anterior and posterior sections and the left hemi-liver into medial and lateral sections; every section has further ramifications into segments and each segment has one or more independent pedicles
[39][40][47,48]. These deep and distal ramifications introduce the concept of the “cone unit”, the smallest resectable anatomical part of the liver, supplied by a tertiary branch and with the base on the hepatic surface and the apex towards the hilum;
[41][49] each segment consists of six to eight cone units, which can all be separately identified by one feeding pedicle and resected as independent anatomical subsegments
[42][50].
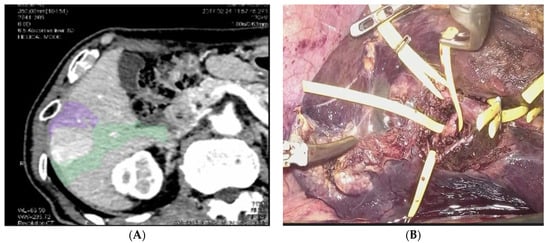
This concept of a precise and deep understanding of the anatomy of the liver, paired with the oncological principle of anatomical resections and with the technological advancements, led some centers to perform what is known as laparoscopic anatomical parenchymal sparing liver resections (
Figure 3)
[25][33][33,41]. This technique requires a careful and detailed preoperative planning and advanced laparoscopic technical skills. Preoperative 3D reconstructions and surgical planning, intraoperative use of contrast ultrasonography, the intraoperative use of the Glissonian approach from the liver hilum and the Indocyanine Green Dye (ICG) negative staining are the basic steps to perform this technique that is hereby summarized.
Figure 3. Anatomical Parenchymal Sparing Liver Resections: (A) 3D reconstruction with tumor bearing area from pedicles of segment 5 and segment 6. (B) Intraoperative view of the isolation of the Glissonian pedicles for segment 5 and 6.