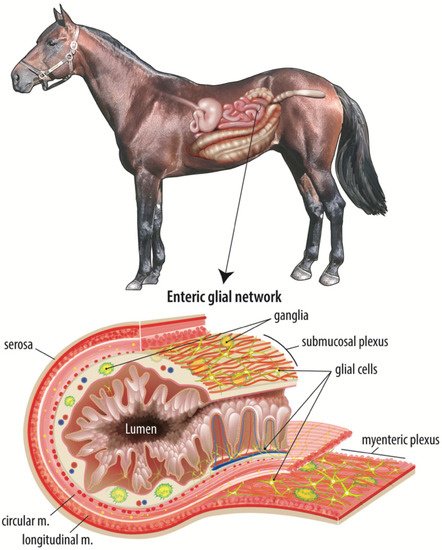
Barrier repair is regulated intensively by complex and coordinated subepithelial cell signaling events. There are many cellular components of the subepithelial microenvironment to consider when investigating age-related differences in intestinal repair. The small intestine is a complex organ comprised of several layers, the outermost being the serosa surrounding the longitudinal and circular muscle layers. The smooth muscle layers facilitate motility, driving segmentation, churning, and forward transit of digesta, while ensuring adequate contact time between the luminal contents and the epithelium to increase absorption of digested nutrients. Between the two layers of smooth muscle is the myenteric plexus of the ENS, a network of intrinsic primary afferent neurons, interneurons, excitatory and inhibitory neurons, and enteric glial cells that regulate smooth muscle activity among many other emerging functions
[11][12][9,35].
Internal to the smooth muscle layers is the submucosa, a region of loose connective tissue containing vasculature, lymphatic vessels, and the submucosal plexus of the ENS, another network of neurons and enteric glial cells (
Figure 1). This region also houses a diverse population of immune cells, mesenchymal cells, and endothelial cells. Intestinal mesenchymal cells consist of fibroblasts, myofibroblasts, pericytes, mesenchymal stem cells, smooth muscle cells, interstitial cells of Cajal, and fibrocytes
[13][36]. These cells function to provide mechanical support, epithelial homeostasis, stem cell maintenance, immune regulation, extracellular matrix maintenance, angiogenesis, and vascular function regulation
[13][36]. Studies have identified a therapeutic effect when inflamed and damaged intestine is treated with mesenchymal stem cells in vitro
[14][15][16][37,38,39]. Endothelial cells form the barrier between the intravascular elements and the submucosal microenvironment and are responsible for the induction of inflammation and recruitment of leukocytes through the release of several proinflammatory cytokines and colony-stimulating factors
[17][40]. The innermost layer is the intestinal mucosa, containing subepithelial capillaries and lymphatic vessels, neuronal projections, and yet another network of enteric glial cells with projections extending close enough to directly contact the single-cell thick epithelial barrier. This epithelial population includes a carefully coordinated and organized population of absorptive and secretory enterocytes, enteroendocrine cells, goblet cells, tuft cells, and intestinal stem cells
[18][41].
Figure 1. The adult equine enteric glial network. The intestinal mucosal microenvironment is home to several cell types that all work in a complex, coordinated manner to maintain the epithelial barrier and restore the barrier in response to intestinal injury. Of these cell types, the enteric glial network is thought to act as an intermediary between the enteric neurons and luminal signals, such as nutrients or microbial metabolites. This figure illustrates the complexity and expanse of the enteric glial network, including directly adjacent to the epithelium.
On the opposite side of the epithelial barrier, the small intestinal lumen contains ingesta, mucus, and two microbial populations, one that is suspended within the ingesta and another that is adherent to the mucus layer between the luminal contents and the mucosa. Within these two populations, microbes can be commensal, symbiotic, or pathogenic, and the intestinal microbiota is increasingly implicated broadly in health and disease
[19][20][42,43]. Mucus is continually secreted by goblet cells in the small intestine and provides both a chemical and physical barrier to the potentially harmful bacteria in the intestinal lumen
[21][44]. Loss of this mucus layer, through ischemic injury for example, increases intestinal permeability and the risk of patient sepsis
[22][45]. Dietary components found in the intestinal lumen also function to stimulate mucus secretion and absorption while other nutrients function to decrease barrier activity. For example, glucose causes intracellular tight junctions to open the paracellular space, allowing nutrients and water to cross the barrier more easily
[23][10]. These components of the luminal microenvironment are equally important to consider when investigating age-dependent mucosal repair following ischemic intestinal injury.