The replacement of damaged or degenerated articular cartilage tissue remains a challenge, as this non-vascularized tissue has a very limited self-healing capacity. Therefore, tissue engineering (TE) of cartilage is a promising treatment option. Although significant progress has been made in recent years, there is still a lack of scaffolds that ensure the formation of functional cartilage tissue while meeting the mechanical requirements for chondrogenic TE. In this article, we report the application of flock technology, a common process in the modern textile industry, to produce flock scaffolds made of chitosan (a biodegradable and biocompatible biopolymer) for chondrogenic TE. By combining an alginate hydrogel with a chitosan flock scaffold (CFS+ALG), a fiber-reinforced hydrogel with anisotropic properties was developed to support chondrogenic differentiation of embedded human chondrocytes. Pure alginate hydrogels (ALG) and pure chitosan flock scaffolds (CFS) were studied as controls. Morphology of primary human chondrocytes analyzed by cLSM and SEM showed a round, chondrogenic phenotype in CFS+ALG and ALG after 21 days of differentiation, whereas chondrocytes on CFS formed spheroids. The compressive strength of CFS+ALG was higher than the compressive strength of ALG and CFS alone. Chondrocytes embedded in CFS+ALG showed gene expression of chondrogenic markers (COL II, COMP, ACAN), the highest collagen II/I ratio, and production of the typical extracellular matrix such as sGAG and collagen II. The combination of alginate hydrogel with chitosan flock scaffolds resulted in a scaffold with anisotropic structure, good mechanical properties, elasticity, and porosity that supported chondrogenic differentiation of inserted human chondrocytes and expression of chondrogenic markers and typical extracellular matrix.
- chitosan
- electrostatic flocking
- chondrocytes
1. Introduction
The aim of this study was to evaluate the suitability of chitosan flock scaffolds (CFS) for cartilage TE for the first time. Flock scaffolds were combined with alginate hydrogels (ALG) and human primary chondrocytes. Chondrogenic (re-) differentiation and thus the suitability of flock scaffolds for cartilage TE was investigated in vitro.
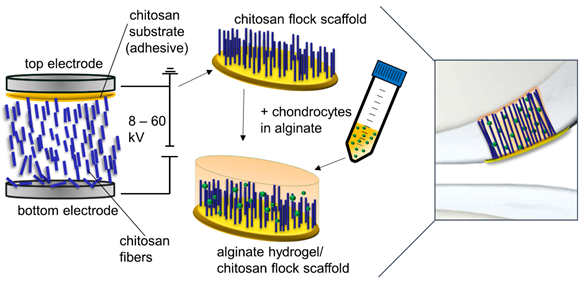
Figure 1
2. Scaffold fabrication and characterisation
Three different types of scaffolds were included in the study: alginate hydrogels (ALG), chitosan flock scaffolds (CFS), and alginate-filled chitosan flock scaffolds (CFS+ALG) (Figure 2). A detailed characterization of the CFS was published before [4]. The CFS used in this study showed a uniform distribution of the fibers with 2 mm length (fiber density 73 ± 8 mm−2) at a mean fiber distance of 149 ± 71 µm. For cell culture, 6 mm diameter scaffolds were punched out. After filling with 1.2% alginate solution in which the cells were suspended and crosslinking with calcium chloride solution, uniformly filled CFS+ALG were obtained (Figure 2B).
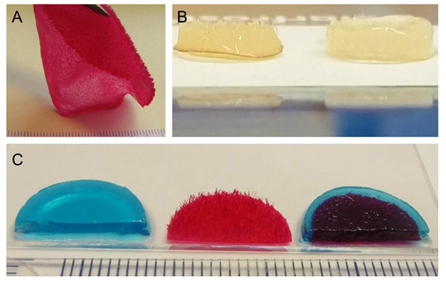
Figure 2
Mechanical characterisation revealed a higher compressive strenght of chitosan flock scaffolds with and without alginate compared to the pure alginate gels.
3. Interaction with cells
Primary human chondrocytes from three donors were introduced into the three types of scaffolds: CFS, ALG, and CFS+ALG. All cell-loaded constructs were cultured under chondrogenic stimulation for 21 days. Figure 3 shows the phenotype of chondrocytes introduced into the three scaffold types. Cells in pure alginate gel retained their round phenotype. After culturing the cells in CFS+ALG, some cells also exhibited a round phenotype in addition to cells with a spindle-shaped elongated cell shape that adhered directly to the chitosan fibers. In contrast, the cells in CFS formed spheroids about 100–200 µm in size, which were distributed over the entire scaffold and were mainly found near the basal chitosan membrane.
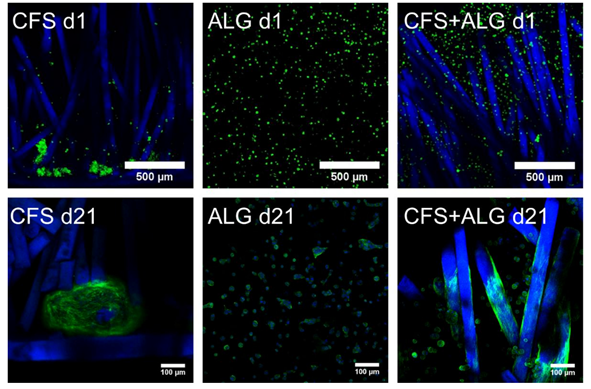
Figure 3
After 21 days of cultivation, the expression of the chondrogenic marker genes aggrecan (ACAN), collagen II (COL II), and cartilage oligomeric ma- trix protein (COMP) was analyzed by quantitative real-time PCR (Figure 4). The expression of collagen I (COL I) as a fibroblast marker and, therefore, for chondrocyte dedifferentiation was also analyzed.
The chondrogenic marker genes COL II and COMP show an increase in expression in all donors and in all scaffold types after 21 days of cultivation. The expression of COL II was highest in CFS+ALG and in ALG compared to chondrocytes cultivated in pure CFS. The expression of aggrecan varied depending on the scaffold type. While, after cultivation in CFS+ALG and ALG, all three donors showed an increase in expression, the expression of ACAN was reduced or only slightly increased after cultivation in CFS.
The collagen II/I ratio, which is an indicator for chondrogenic differentiation without formation of fibrocartilage, was highest in CFS+ALG scaffolds (Figure 4).
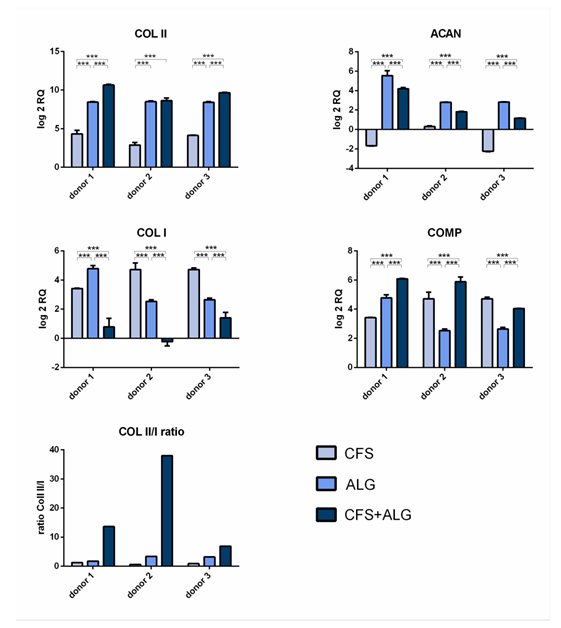
Figure 4
The secretion of sulfated glycosaminoglycans (sGAG) as well as the secretion of collagen II was higher for chondrocytes cultured in CFS+ALG compared to CFS or ALG alone (Figure 5).
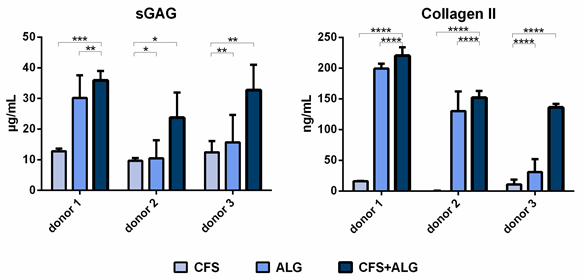
Figure 5
4. Discussion
The articular cartilage is anisotropic and inhomogeneous due to its microstructure. Its elastic properties are influenced by the arrangement of collagen fibers, which are oriented orthogonally to the bone–cartilage interface in the deep zone, randomly in the middle zone, and parallel to the surface in the superficial zone [5][2]. This anisotropy and associated resistance to loading is induced by the fiber alignment in the scaffold, which is also anisotropic as a result. Such an ordered structure can guide chondrocytes to produce their own extracellular matrix and thus fill a defect with functional tissue. Flocking creates an organized structure due to the vertical and parallel alignment of the fibers, resulting in an anisotropic scaffold with suitable mechanical stability and high elasticity.
The low mechanical strength of pure alginate hydrogels, which was confirmed in our results, impairs their application for tissue engineering of cartilage, where high overall mechanical strength is required. However, mechanical strength of alginate hydrogels was significantly increased by combining it with a CFS, and the combination even exceeded the compressive strength of the pure flock scaffold. While in pure flock scaffolds, the high compressive strength is mainly due to the progressive elastic behavior of the fibers and the increased fiber-volume fraction as well as fiber-to-fiber contacts with increasing compression [6], combined scaffolds further benefit from the restricting gel matrix, which may decrease bending and buckling tendencies in individual embedded fibers and make the combined scaffold act more like a typical fiber-reinforced composite.
Embedding of flock scaffolds into crosslinked alginate hydrogels results in fiber- reinforced hydrogels. Fiber reinforcement is a strategy to overcome the disadvantage of low stiffness of hydrogels while retaining their high water binding capacity and porosity at the same time [7][8][9]. The opposite electric charge of chitosan and alginate may also play a role in the increased compressive strength of the CFS+ALG, which is mediated by the negatively charged carboxylate groups of alginate and the positively charged amino groups of chitosan [10].
It is known that cells embedded in alginate hydrogels maintain a round phenotype, which is important for the redifferentiation of the introduced chondrocytes, as they lose their round chondrogenic phenotype during two-dimensional in vitro expansion in monolayer culture [11]. In our study, the phenotype of the introduced cells differed in the three scaffold types. In the combined CFS+ALG, few cells adhered to the chitosan fibers with a fibroblast-like phenotype oriented along the fibers. Cell adhesion to chitosan was described for different cell types such as human bone mesenchymal stem cells (hBMSC), human adipose-tissue-derived stem cells, neurons, and fibroblasts [4][12][13][14][15] and depends on the degree of deacetylation of chitosan. Due to the positively charged amino groups and hydrophilic surface, chitosan enables interactions with the anionic cell surface and anionic GAGs, proteoglycans, and other negatively charged matrix molecules. In contrast to the alginate-containing scaffolds, the chondrocytes which were introduced into the pure CFS formed cellular agglomerates 24 h after seeding and large spheroids within 21 days. Cell aggregation and round morphology have previously been related to a pro-chondrogenic phenotype, and cellular aggregates enhance the chondrogenic differentiation ability of cells. It was previously shown that among other cell types, hBMSC and adipose-tissue-derived stem cells (hADSC) could self-organize into 3D spheroids with higher chondrogenic differentiation capacity when cultured on chitosan [16][17][18][19]. In our study, spheroid formation may have been favored by cell aggregation and spatial proximity after seeding on the membrane because cell–cell contacts dominate over cell–matrix interactions in the formation of spheroids. In the CFS+ALG, cell–matrix contacts and cell–cell contacts are limited by immobilization. In vitro cultivation conditions of chondrocytes also influence the composition of the newly secreted extracellular matrix. In natural cartilage tissue, viscoelastic properties result from the structure and composition of this extracellular matrix, whose main components are proteoglycans and collagen II, both being arranged in a highly organized manner. Since 2D cultivation or expansion leads to dedifferentiation of chondrocytes [11][20], it is important that the cultivation conditions sustain redifferentiation with a chondrocyte-typical matrix. Alginate hydrogels have already been shown to support the growth and proliferation of encapsulated chondrocytes, as well as maintain their chondrogenic phenotype and lead to the expression of chondrogenic markers such as aggrecan, COMP, and sGAG, as well as collagen II [21][22][23].
The highest expression of collagen II, cartilage oligomeric matrix protein, and sGAG was found after culturing chondrocytes in the CFS+ALG. At the same time, the expression of collagen I was lowest in these combined scaffolds. Collagen I is associated with a fibrocartilaginous and dedifferentiated cell type of chondrocytes and is observed to be increased after two-dimensional culturing for expansion or differentiation [11][22][20]. However, in line with our results, Caron et al. [22] described that collagen I is also induced upon three-dimensional cultivation of chondrocytes in alginate gels and cell pellets.
While collagen II expression at mRNA and protein levels of the chondrocytes cultivated in the CFS+ALG composite was similar to that in pure alginate, the low collagen I expression detected in CFS+ALG composites leads to a significantly increased collagen II/I ratio compared to both pure chitosan and alginate constructs. Marlovits et al. [24] reported that this ratio is above 400 at the mRNA level in freshly isolated chondrocytes and the beginning of cultivation and decreases to values between 0.1 and 1 during monolayer cultivation over 30 days. During cultivation in three-dimensional matrices, the expres- sion of collagen II increases, while that of collagen I is decreasing; in our studies, the mRNA collagen II/I ratio showed a maximum of 37 after 21 days of redifferentiation of chondrocytes cultivated in CFS+ALG.
Chondrocytes cultured in pure CFS showed the lowest expression of collagen II at the RNA and protein levels. Collagen II is important for chondrogenic differentiation, and it prevents hypertrophy of chondrocytes and supports the formation of cell–matrix contacts [25][26]. Although it is known that culturing chondrocytes in pellets or spheroids facilitates redifferentiation and is used to form hyaline-like cartilage [22][27] in our study, the differentiation of cells that formed spheroids in the CFS was low based on analyses on both RNA and protein levels.
Several studies have focused on the expression of chondrogenic markers in scaffolds and hydrogels made of chitosan and alginate and have obtained heterogeneous results in detail. Li and Zhang [28] found a higher collagen II expression of chondrocytes in combined chitosan/alginate freeze-dried sponges compared to the expression of HTB94 in pure chitosan freeze-dried sponges. In vivo analyses of cell-laden alginate and chitosan hydrogels suggest higher suitability of chitosan for chondral tissue engineering since it retained the highest amount of sGAG and did not promote vascularization or endochondral ossification [29]. However, the results are only comparable to a limited extent due to the different types of scaffolds. Research of chondrogenic differentiation markers in combined chitosan/alginate scaffolds [10] showed that cartilaginous matrix proteins such as collagen type II, GAG, and aggrecan are produced when chondrocytes are cultured in these materials. Although these freeze-dried sponges, as well as pure alginate hydrogels, promoting the maintenance of the chondrogenic cell type, these scaffold types have low mechanical strength and degrade rapidly in physiological environments.
Especially in tissue engineering of cartilage, it is of particular importance to produce a graft that can withstand the multiple forces to which cartilage is subjected. The combination of alginate hydrogels with axially oriented chitosan fibers results in a mechanically stable anisotropic scaffold that can be more resistant to compressive loads perpendicular to the fiber orientation and has a higher elasticity compared to both the pure alginate hydrogels and flock scaffolds. Besides the promising properties of CFS in terms of biocompatibility, porosity, anisotropic morphology, and mechanical stability, these scaffolds were shown to support chondrogenic redifferentiation in our study. Differentiation of chondrocytes in an alginate hydrogel combined with a chitosan flock scaffold was superior to the pure alginate gel and pure chitosan flock scaffolds. By combining an alginate gel with its known advantages for chondrogenic differentiation with a chitosan flock scaffold, the disadvantages of the mechanical properties of a pure hydrogel can be overcome, and support of chondrogenic differentiation can be further improved. Further studies will be helpful to strengthen the evidence of this study, which is partially limited due to the small number of chondrocyte donors, the pathology of the donors, and the limited selection of chondrogenic markers. Moreover, further studies will involve in vivo testing of the constructs in an animal model.
References
- Davis, S.; Roldo, M.; Blunn, G.; Tozzi, G.; Roncada, T. Influence of the Mechanical Environment on the Regeneration of Osteochondral Defects. Front. Bioeng. Biotechnol. 2021, 9, 603408.
- Armiento, A.R.; Stoddart, M.J.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20.
- Jiang, S.; Guo, W.; Tian, G.; Luo, X.; Peng, L.; Liu, S.; Sui, X.; Guo, Q.; Li, X. Clinical Application Status of Articular Cartilage Regeneration Techniques: Tissue-Engineered Cartilage Brings New Hope. Stem. Cells Int. 2020, 2020, 1–16.
- Gossla, E.; Tonndorf, R.; Bernhardt, A.; Kirsten, M.; Hund, R.-D.; Aibibu, D.; Cherif, C.; Gelinsky, M. Electrostatic flocking of chitosan fibres leads to highly porous, elastic and fully biodegradable anisotropic scaffolds. Acta Biomater. 2016, 44, 267–276.
- Steck, E.; Bertram, H.; Walther, A.; Brohm, K.; Mrozik, B.; Rathmann, M.; Merle, C.; Gelinsky, M.; Richter, W. Enhanced Biochemical and Biomechanical Properties of Scaffolds Generated by Flock Technology for Cartilage Tissue Engineering. Tissue Eng. Part A 2010, 16, 3697–3707.
- Tonndorf, R.; Gossla, E.; Kocaman, R.T.; Kirsten, M.; Hund, R.-D.; Hoffmann, G.; Aibibu, D.; Gelinsky, M.; Cherif, C. Factors affecting the mechanical and geometrical properties of electrostatically flocked pure chitosan fiber scaffolds. Text. Res. J. 2018, 88, 1965–1978
- Critchley, S.; Sheehy, E.J.; Cunniffe, G.; Diaz-Payno, P.; Carroll, S.F.; Jeon, O.; Alsberg, E.; Brama, P.A.J.; Kelly, D.J. 3D printing of fibre-reinforced cartilaginous templates for the regeneration of osteochondral defects. Acta Biomater. 2020, 113, 130–143.
- Zhang, R.; Wu, Y.; Lin, P.; Jia, Z.; Zhang, Y.; Liu, F.; Yu, B.; Zhou, F. Extremely Tough Hydrogels with Cotton Fibers Reinforced. Adv. Eng. Mater. 2020, 93, 2000508.
- Schipani, R.; Scheurer, S.; Florentin, R.; Critchley, S.E.; Kelly, D.J. Reinforcing interpenetrating network hydrogels with 3D printed polymer networks to engineer cartilage mimetic composites. Biofabrication 2020, 12, 35011.
- Reed, S.; Wu, B.M. Biological and mechanical characterization of chitosan-alginate scaffolds for growth factor delivery and chondrogenesis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 272–282.
- von der Mark, K.; Gauss, V.; von der Mark, H.; Müller, P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature 1977, 267, 531–532.
- Huang, H.; Zhang, X.; Hu, X.; Dai, L.; Zhu, J.; Man, Z.; Chen, H.; Zhou, C.; Ao, Y. Directing chondrogenic differentiation of mesenchymal stem cells with a solid-supported chitosan thermogel for cartilage tissue engineering. Biomed. Mater. 2014, 9, 35008.
- Huang, Y.; Seitz, D.; Chevalier, Y.; Müller, P.E.; Jansson, V.; Klar, R.M. Synergistic interaction of hTGF-β3 with hBMP-6 promotes articular cartilage formation in chitosan scaffolds with hADSCs: Implications for regenerative medicine. BMC Biotechnol. 2020, 20, 48.
- Carvalho, C.R.; López-Cebral, R.; Silva-Correia, J.; Silva, J.M.; Mano, J.F.; Silva, T.H.; Freier, T.; Reis, R.L.; Oliveira, J.M. Investigation of cell adhesion in chitosan membranes for peripheral nerve regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 1122–1134.
- Freier, T.; Koh, H.S.; Kazazian, K.; Shoichet, M.S. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials 2005, 26, 5872–5878.
- Gionet-Gonzales, M.A.; Leach, J.K. Engineering principles for guiding spheroid function in the regeneration of bone, cartilage, and skin. Biomed. Mater. 2018, 13, 34109.
- Lu, T.-J.; Chiu, F.-Y.; Chiu, H.-Y.; Chang, M.-C.; Hung, S.-C. Chondrogenic Differentiation of Mesenchymal Stem Cells in Three-Dimensional Chitosan Film Culture. Cell Transpl. 2017, 26, 417–427.
- Yeh, H.-Y.; Hsieh, F.-Y.; Hsu, S.-h. Self-patterning of adipose-derived mesenchymal stem cells and chondrocytes cocultured on hyaluronan-grafted chitosan surface. Biointerphases 2016, 11, 11011.
- Huang, G.-S.; Dai, L.-G.; Yen, B.L.; Hsu, S.-h. Spheroid formation of mesenchymal stem cells on chitosan and chitosan-hyaluronan membranes. Biomaterials 2011, 32, 6929–6945.
- Layman, D.L.; Sokoloff, L.; Miller, E.J. Collagen synthesis by articular chondrocytes in monolayer culture. Exp. Cell Res. 1972, 73, 107–112.
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25.
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentia-
- Guo, J.F.; Jourdian, G.W.; MacCallum, D.K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect. Tissue Res. 1989, 19, 277–297.
- Marlovits, S.; Hombauer, M.; Truppe, M.; Vècsei, V.; Schlegel, W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Jt. Surg. Br. 2004, 86, 286–295.
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1-SMAD1 interaction. Bone Res. 2019, 7, 8.
- Choi, B.; Kim, S.; Lin, B.; Wu, B.M.; Lee, M. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 20110–20121.
- Martinez, I.; Elvenes, J.; Olsen, R.; Bertheussen, K.; Johansen, O. Redifferentiation of In Vitro Expanded Adult Articular Chondrocytes by Combining the Hanging-Drop Cultivation Method with Hypoxic Environment. Cell Transpl. 2008, 17, 987–996.
- Li, Z.; Zhang, M. Chitosan-alginate as scaffolding material for cartilage tissue engineering. J. Biomed. Mater. Res. A 2005, 75, 485–493
- Sheehy, E.J.; Mesallati, T.; Vinardell, T.; Kelly, D.J. Engineering cartilage or endochondral bone: A comparison of different naturally derived hydrogels. Acta Biomater. 2015, 13, 245–253
- Marlovits, S.; Hombauer, M.; Truppe, M.; Vècsei, V.; Schlegel, W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Jt. Surg. Br. 2004, 86, 286–295.
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin β1-SMAD1 interaction. Bone Res. 2019, 7, 8.
- Choi, B.; Kim, S.; Lin, B.; Wu, B.M.; Lee, M. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl. Mater. Interfaces 2014, 6, 20110–20121.
- Martinez, I.; Elvenes, J.; Olsen, R.; Bertheussen, K.; Johansen, O. Redifferentiation of In Vitro Expanded Adult Articular Chondrocytes by Combining the Hanging-Drop Cultivation Method with Hypoxic Environment. Cell Transpl. 2008, 17, 987–996.
- Sheehy, E.J.; Mesallati, T.; Vinardell, T.; Kelly, D.J. Engineering cartilage or endochondral bone: A comparison of different naturally derived hydrogels. Acta Biomater. 2015, 13, 245–253.
- Tonndorf, R.; Gossla, E.; Kocaman, R.T.; Kirsten, M.; Hund, R.-D.; Hoffmann, G.; Aibibu, D.; Gelinsky, M.; Cherif, C. Factors affecting the mechanical and geometrical properties of electrostatically flocked pure chitosan fiber scaffolds. Text. Res. J. 2018, 88, 1965–1978
- Caron, M.M.J.; Emans, P.J.; Coolsen, M.M.E.; Voss, L.; Surtel, D.A.M.; Cremers, A.; van Rhijn, L.W.; Welting, T.J.M. Redifferentia-
- tion of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178
- Li, Z.; Zhang, M. Chitosan-alginate as scaffolding material for cartilage tissue engineering. J. Biomed. Mater. Res. A 2005, 75,
- Sheehy, E.J.; Mesallati, T.; Vinardell, T.; Kelly, D.J. Engineering cartilage or endochondral bone: A comparison of different naturally derived hydrogels. Acta Biomater. 2015, 13, 245–253
