Metabolic syndrome (MetS) is a non-communicable disease characterised by a cluster of metabolic irregularities. The prevalence of MetS in people living with Human Immunodeficiency Virus (HIV) and antiretroviral (ARV) usage is increasing rapidly. The findings suggest that mitochondrial dysfunction was the most common mechanism that induced metabolic complications. Furthermore, protease inhibitors (PIs) are more commonly implicated in MetS-related effects than other classes of ARVs.
- metabolic syndrome
- HIV
- ARVs
- mitochondrial dysfunction
- inflammation
1. Introduction
2. HIV and MetS
Many risk factors contribute to the incidence of MetS, including an unhealthy diet, lack of exercise, and age [6][16]. However, research has established a unique set of risk factors associated with HIV infection [7][17]. The common risk factors highlighted in HIV infection include chronic inflammation and immune dysfunction, which promotes atherosclerosis, dyslipidaemia, and T2DM [8][18]. The biochemical basis of HIV-induced MetS remains elusive; however, research has established a few common factors. HIV can induce MetS via several mechanisms (Figure 1). The most common is via the activation of inflammatory responses, cellular apoptosis, and mitochondrial dysfunction. However, epigenetic modifications are emerging in recent research surrounding HIV and inflammation. The ability to induce the aforementioned pathways and changes leads to more severe consequences, such as insulin resistance, dyslipidaemia, and obesity [9][2].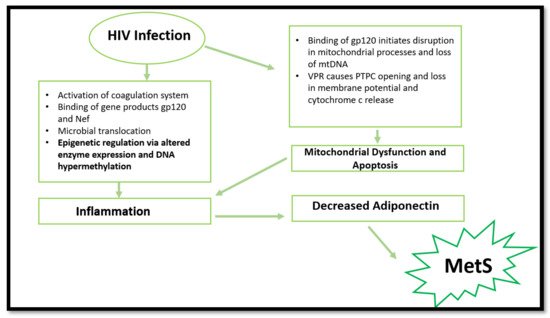
3. HIV, Mitochondrial Dysfunction, and Cell Apoptosis
4. HIV and Epigenetic Modifications
Aside from the common mechanisms, more recent research has suggested that HIV-1 infection can cause epigenetic changes when exposed to Mycobacterium tuberculosis. The latter results in altered monocyte function and dysregulation in pro-inflammatory cytokine production. The same study suggested that a decrease in global DNA methylation occurred in HIV-infected individuals. This was mainly attributed to the downregulation of DNA methyltransferases and the upregulation of methyl-CpG-binding proteins. Consequently, the reduction in global DNA methylation caused an increased activation status of monocytes. This result was accompanied by increased production of pro-inflammatory cytokines [16][45]. These findings are significant, considering the vulnerability to Mycobacterium tuberculosis in developing countries with high HIV prevalence, such as South Africa. The study provides plausible cause to initiate future in vivo research that will aid in understanding epigenetic changes associated with HIV and MetS.
More recent findings report DNA hypermethylation in HIV-infected patients. These epigenetic modifications result in cell dysfunction and decreased cytokine production, thus increasing CVD and T2DM risk [17][46]. More specifically, the HIV-1 infection causes epigenetic changes in the T-cell population, resulting in aberrant expression of pro-inflammatory cytokines and immune-related genes [18][47]. This is caused by the virus inducing DNA methylation changes in essential genes (IL-2, PD-1 and FOXP3) in T-cells which initiates cell dysfunction [19][20][21][48,49,50].
5. The Evolution of ARVs and Implications in MetS
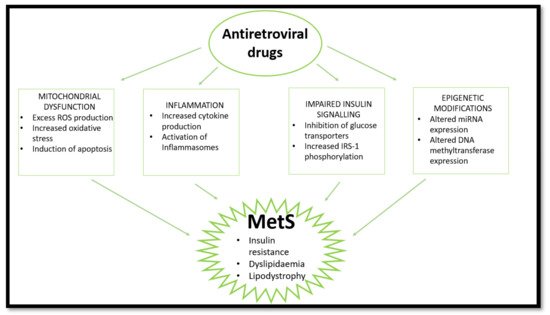
The use of ARVs provides temporary relief for HIV-induced effects but has induced various complications over time (Masuku et al., 2019). Side effects associated with the use of ARVs have improved following extensive research. Previously, adverse outcomes seen with ARV usage resulted in patients discontinuing treatment or switching combinations of drugs until the side effects were manageable. This was mainly observed in first- and second-generation treatment (O’Brien et al., 2003). Initially, the discovery of ARVs led to various trials of singular and combinational usage, with NNRTIs and NRTIs being popular. However, the usage of the earlier generations of ARVs were later associated with adversity and HIV drug resistance (WHO, 2020). Fortunately, the newer generation of ARVs are linked with fewer complications and adversity (Barnhart and Shelton, 2015; Rai et al., 2018). More specifically, the World Health Organization has highlighted the need to move to newer generations of ARVs such as dolutegravir to prevent the HIV drug resistance associated with former generations and reduce side effects (WHO, 2020). However, some problems still arise following the usage of the current generation of ARVS. Long-term use of ARVs is associated with the development of MetS through induction of dyslipidaemia, lipodystrophy, mitochondrial dysfunction, and insulin resistance [22][51]. Over time, metabolic dysregulation occurs, initiating changes in fat distribution and glucose homeostasis [23][52]. The consensus in population studies indicates a high prevalence of MetS in PLWH receiving ARV treatment [3][24][25][9,53,54]. This review looks at the effects of nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and integrase strand transfer inhibitors (INSTIs) on mitochondrial dysfunction, insulin resistance, inflammation, and lipodystrophy/dyslipidaemia which are common markers and outcomes of MetS (Figure 2). Currently, the literature indicates that PIs are most commonly implicated in MetS cases.