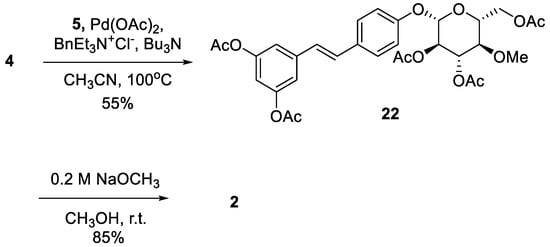
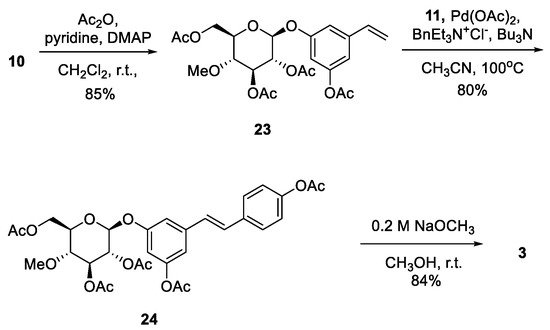
Resveratrol is a well-known dietary polyphenol because it has a variety of beneficial biological activities. The fungus Beauveria bassiana is one of the most frequently used microorganisms for the biotransformation of polyphenols. Recently, resvebassianol A (2), a glycosylated metabolite of resveratrol by B. bassiana, was isolated and structurally elucidated. It was demonstrated to exhibit antioxidant, regenerative, and anti-inflammatory activities with no cytotoxicity. Here, we report the first total synthesis of resvebassianol A, 4'-O-β-(4'"-O-methylglucopyranosyl)resveratrol (2), and its regiomer, 3-O-β-(4 -O-methylglucopyranosyl)resveratrol (3). Key reactions include (i) the construction of a stilbene core via a novel Heck reaction of aryl halides and styrenes, and (ii) glycosylation with unnatural methylglucopyranosyl bromide. The glycosylation step was carefully optimized by varying the bases and solvents. Resveratrol metabolites 2 and 3 were obtained at 7.5% and 6.3% of the overall yield, respectively.
1. Introduction
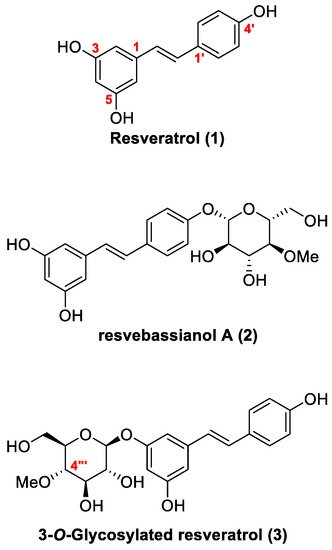
Resveratrol (
1,
trans-3,5,4-trihydroxystilbene) is an important dietary polyphenol and naturally occurring phytoalexin found in grapes, red wine, berries, peanuts, olive oil, etc.
[1][2][3]. It is produced by plants in response to environmental stress and fungal attack through the induction of resveratrol synthetase
[4][5]. Resveratrol was first isolated from the roots of the white hellebore lily (
Veratrum grandiflorum O. Loes) in 1940
[6]. Most of the biological activities of resveratrol have been shown by its
trans stilbene isomer, while the
cis stilbene isomer also occurs naturally
[7]. Resveratrol exerts numerous biological activities such as antioxidant, anti-infective, anti-inflammatory, anti-ischemic, cardioprotective, neuroprotective, anti-aging, anti-viral, anti-obesity, and anti-cancer effects
[8][9][10][11][12][13][14][15][16][17][18]. Recently, it was revealed that its ability to activate various deacetylase enzymes (sirtuins) could be responsible for the various biological properties and delay aging
[19][20].
Despite their pharmacological activities, various in vivo studies have shown that the potential of polyphenols is impaired by their insolubility in water, ultraviolet light instability, poor intestinal absorption, short half-life, rapid clearance, low bioavailability, and rapid metabolism
[21][22]. The introduction of a glycosyl moiety on polyphenols not only helps to enhance the solubility of substrates but also reduces their toxicity, which ultimately increases the activity of biosynthetic intermediates
[23]. Moreover, the sugar moiety of polyphenol glycosides might play a major role in their absorption, resulting in an acceptable concentration in the circulatory streams
[24]. Polyphenols are subjected to enzymatic oxidation by polyphenol oxidases in plants, during food processing, and also after human consumption, which can be protected by glycosylation
[25]. The incorporation of sugar moieties into different types of pharmacophores, natural products, or prodrugs has been proven to improve anti-cancer activities
[26].
Several glycosyl derivatives of resveratrol have been recognized in the roots of
Poligonum cuspidatum such as piceid (3-
O-
β-D-glucosyl resveratrol), resveratroloside (4′-
O-
β-D-glucosylresveratrol), and 4′-
O-
β-D-glucosyl piceatannol
[27]. Piceid has been shown to exhibit a broad range of biological activities
[28].
The fungus
Beauveria bassiana is the most frequently used biocatalyst and has been used to transform more than 300 bioactive compounds
[29][30]. For instance,
B. bassiana ATCC 7159 has been used for the biotransformation of curvularin and kaempferol, leading to the production of new metabolites resulting from 4-
O-methyl glucosylation of the substrate, and was highly selective among different hydroxyl groups in the same molecule
[30]. Recently, resvebassianol A (
2) shown in
Figure 1, was identified through biotransformation of resveratrol by
B. bassiana and exhibited important pharmacological activities such as inhibition of inflammatory cytokine expression and cell rejuvenation. Moreover, compared with resveratrol, resvebassianol A proved to be less toxic and more stable
[31].
Figure 1. Chemical structures of resveratrol 1, resvebassianol A (2), and 3-O-β-(4‴-O-methylglucopyranosyl) resveratrol 3.
Several synthetic approaches for the formation of glycosidic bonds to phenolic OH in resveratrol have been reported. Direct coupling of resveratrol with a bromo-glucuronide donor was performed by Wang et al. for the synthesis of two glycoconjugates
[32]. Coupling of resveratrol with glucuronyl bromide was performed using silver carbonate as an activator, in order to produce glucuronide-conjugated resveratrol in low yield, possibly due to the low solubility of resveratrol in organic solvents. Lucas et al. synthesized resveratrol 3-
O-
β-D-glucuronide by coupling a trichloroacetimidate glycosyl donor with protected resveratrol using TMSOTf and BF
3.OEt
2 as promoters
[3]. Learmonth also synthesized two glucuronide conjugates of resveratrol, in which palladium-catalyzed Heck coupling of an iodo-
O-
β-D-glucuronate derivative and its corresponding styrene was adopted
[33].
The structural uniqueness and natural resource scarcity of resvebassianol A for biological evaluation prompted us to develop an efficient synthetic method for the metabolite. In this study, we report the total synthesis of resvebassianol A (
2), a metabolite of resveratrol by
B. bassiana, and its regiomer, 3-
O-
β-(4‴-
O-methylglucopyranosyl)resveratrol (
3).
2. Results and Discussion
2.1. Retrosynthesis
Metabolite
2 and its regiomer
3 consist of a glycone attached to the aglycone moiety. The glycone moiety is 4-
O-methyl glucopyranose, whereas the aglycone is a functional resveratrol featuring a stilbene core with a polyhydroxy group. Metabolite
2 is a structure in which 4-
O-methylglucopyranose is attached to the 7-position hydroxyl group of resveratrol, whereas its regiomer
3 consists of a glycosyl moiety attached to the 3-position hydroxyl group of resveratrol. The synthesis of both metabolites involves a glycosylation reaction that introduces methylated glucose as the core reaction and the Heck reaction to form a stilbene skeleton
[34].
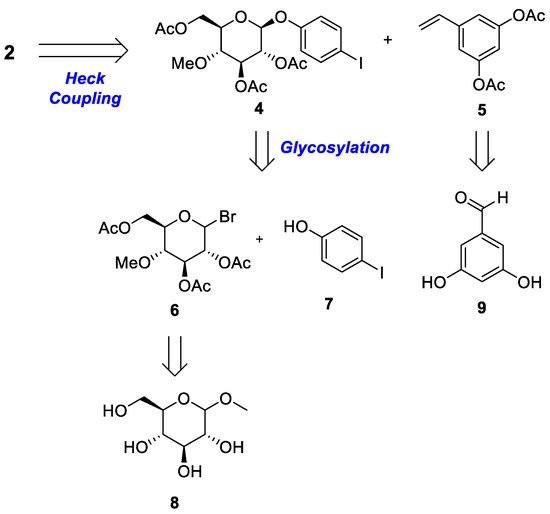
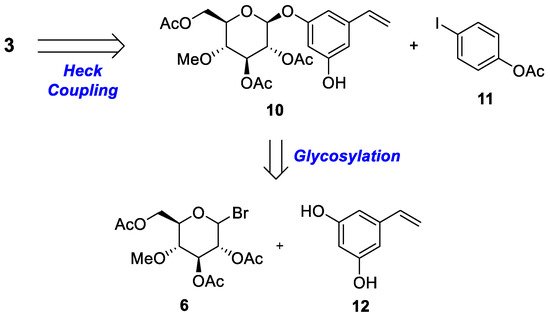
Scheme 1 and
Scheme 2 provide a retrosynthetic methodology for the synthesis of both metabolites
2 and
3. Stilbene moiety
2 and its regiomer
3 were constructed via palladium-catalyzed Heck coupling. The rate-limiting step of the glycosylation reaction was performed with selectively protected compound
6 and commercially available iodophenol
7. Compound
10 was obtained from the glycosylation of compound
6 and styrene
12, which was synthesized from readily available dihydroxy benzaldehyde
9.
Scheme 1. Retrosynthetic analysis of 4′-O-β- (4‴-O-methylglucopyranosyl)resveratrol 2.
Scheme 2. Retrosynthetic analysis of 3-O-β-(4‴-O-methylglucopyranosyl)resveratrol 3.
2.2. Chemistry
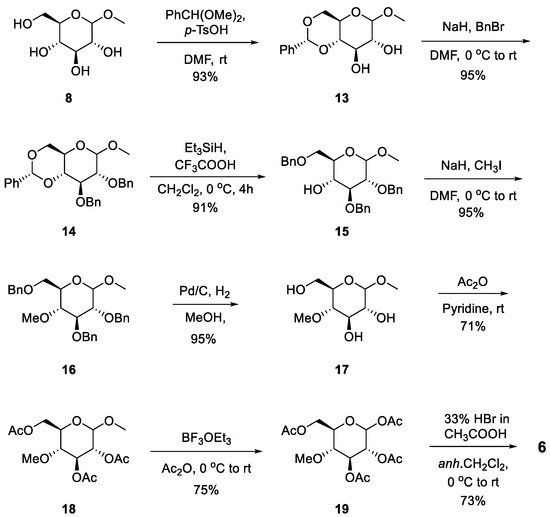
The reaction commenced with the preparation of the glycosyl donor, 4-O-methylglycopyranosyl bromide
6, as shown in
Scheme 3, which involves eight steps from commercially available methyl-α-D-glucopyranoside
8. Regioselective protection of 4, 6-diol from the starting material was accomplished by the introduction of a 4,6-O-benzylidene group using benzaldehyde dimethyl acetal under acidic conditions, yielding protected compound
13. 2,3-Di-O-benzylation of
13 generated
14 using NaH and BnBr. Further regioselective opening of the benzylidene ring of intermediate
14 was conducted with the help of triethylsilane (TES) and trifluoroacetic acid (TFA) to obtain alcohol
15 [35]. Methylation of compound
15 with NaH and MeI in N, N-dimethylformamide yielded product
16, followed by hydrogenolysis to yield product
17. Acetylation of the hydroxy groups of
17 was performed using pyridine and acetic anhydride to yield
18, followed by the replacement of an anomeric methoxy group with an acetoxy group using boron trifluoride diethyl etherate to yield
19. Finally, grafting of the anomeric acetoxy group was performed to incorporate bromine using HBr (33% in acetic acid) to yield acylated glycosyl bromide
6 at 73%
[36]. As the final compound, 4-O-methylglucopyranosyl bromide
6, has poor chemical stability, it is suitable to obtain a large amount of acetate compound
19 and synthesize
6 immediately when necessary.
Scheme 3. Synthesis of 4-O-methylglucopyransyl bromide 6.
3,5. Dihydroxystyrene
12 and 3,5 diacetoxyystyrene
5 were synthesized from commercially available 3,5-dihydroxy benzaldehyde
9 according to
Scheme 4. Protection of the hydroxy group of
9 with TBDMS yielded
20, and the Wittig reaction yielded olefin
21 using methyltriphenylphosphonium bromide under basic conditions. The TBDMS group in intermediate
21 was removed using tetrabutylammonium fluoride (TBAF) to furnish dihydroxy styrene
12, and further acetylation of both hydroxy groups resulted in
12 at 70% yield.
Scheme 4. Synthesis of diacetoxystyrene 5 and dihydroxystyrene 12.
After obtaining
6 and
12, the next target was to synthesize substrates
4 and
10, which participate in the Heck reaction for the synthesis of the stilbene core.
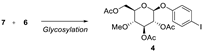
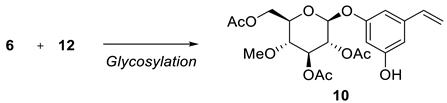
We performed glycosylation of both iodophenol
7 and dihydroxystyrene
12 separately with 4-
O-methylglycopyranosyl bromide
6 under different reaction conditions, as shown in
Table 1 and
Table 2. Using Ag
2CO
3 in acetonitrile produced low-yield glycoside products
4 and
10 up to 16% and 19%, respectively. We attempted to improve glycosylation using a phase transfer catalyst (TBAB) in a two-phase system (aqueous NaOH and K
2CO
3) in CHCl
3. Unfortunately, the reaction yielded trace amounts. The reaction was incomplete, and the substrate was recovered for reuse. Bromide compound
6 can be decomposed into glycal by an alkaline water phase and phenoxide anion
[37]. Therefore, excess use of water in the reaction lowers the yield of the compound. After utilizing several conditions (
Table 1 and
Table 2), the glycosylation reaction under the phase transfer catalyst BnNBu
3Cl and K
2CO
3 as a base at room temperature yielded product
4 at 57% yield. The desired mono-glucosylated product
10 was obtained at 40% yield along with the undesired di-glucosylated product as a mixture, which was separated by column chromatography.
Table 1. Optimization of glycosylation reaction for synthesis of 4 a.
| Entry a |
Base |
Reagent |
Solvent |
Temp. (°C) |
Yield (%) d |
| 1 |
|
Ag2CO3 (1eq) |
CH3CN |
r.t |
16 |
| NaOH |
TBAB |
| 3 |
| r.t |
57 |