Tumor necrosis factor (TNF) is used as a pro-inflammatory payload to trigger haemorrhagic necrosis and boost anti-cancer immunity at the tumor site. There is a depotentiated version of TNF (carrying the single point mutation I97A), which displayed reduced binding affinity to its cognate receptor tumor necrosis factor receptor 1 (TNFR-1) and lower biocidal activity.
- tumor targeting
- immunocytokines
- activity-on-demand
- targeted-TNF
- EDB-fibronectin
1. Introduction
Cytokines are small proteins which modulate the activity of the immune system in health and in disease. Certain proinflammatory cytokines are used for cancer immunotherapy [1][2], while other cytokines with anti-inflammatory properties may be considered as tools for the treatment of chronic inflammatory and autoimmune conditions [3].Targeting an active cytokine to a tumor-associated antigen by means of a fusion with a suitable monoclonal antibody may represent a strategy for the enhancement of the cytokines’ therapeutic index [4][5].
2. Production and Characterization of L19-TNF Mutant Fusion Proteins
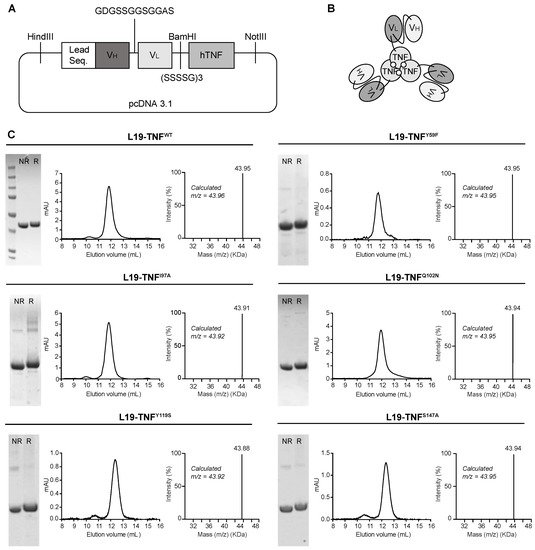
3. Conditional In Vitro Cytotoxicity Effect of L19-TNF Mutants upon EDB Concentration

| No EDB Coating (M) | 100 nM EDB Coating (M) | |
|---|---|---|
| L19-TNFWT | 1.076 × 10−10 | 1.033 × 10−10 |
| L19-TNFY59F | 4.172 × 10−10 | 9.501 × 10−10 |
| L19-TNFI97A | 2.273 × 10−9 | 2.254 × 10−10 |
| L19-TNFQ102N | 5.012 × 10−11 | 5.986 × 10−11 |
| L19-TNFY119S | 3.245 × 10−11 | 6.417 × 10−11 |
| L19-TNFS147A | 1.261 × 10−11 | 1.58 × 10−11 |
4. The Mutant I97A Has Reduced Binding Affinity towards TNFR1
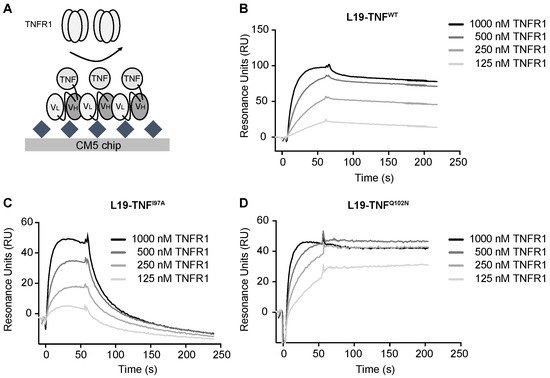
5. The I97A Mutant Accumulates at the Tumor Site and Shows High Levels in Blood
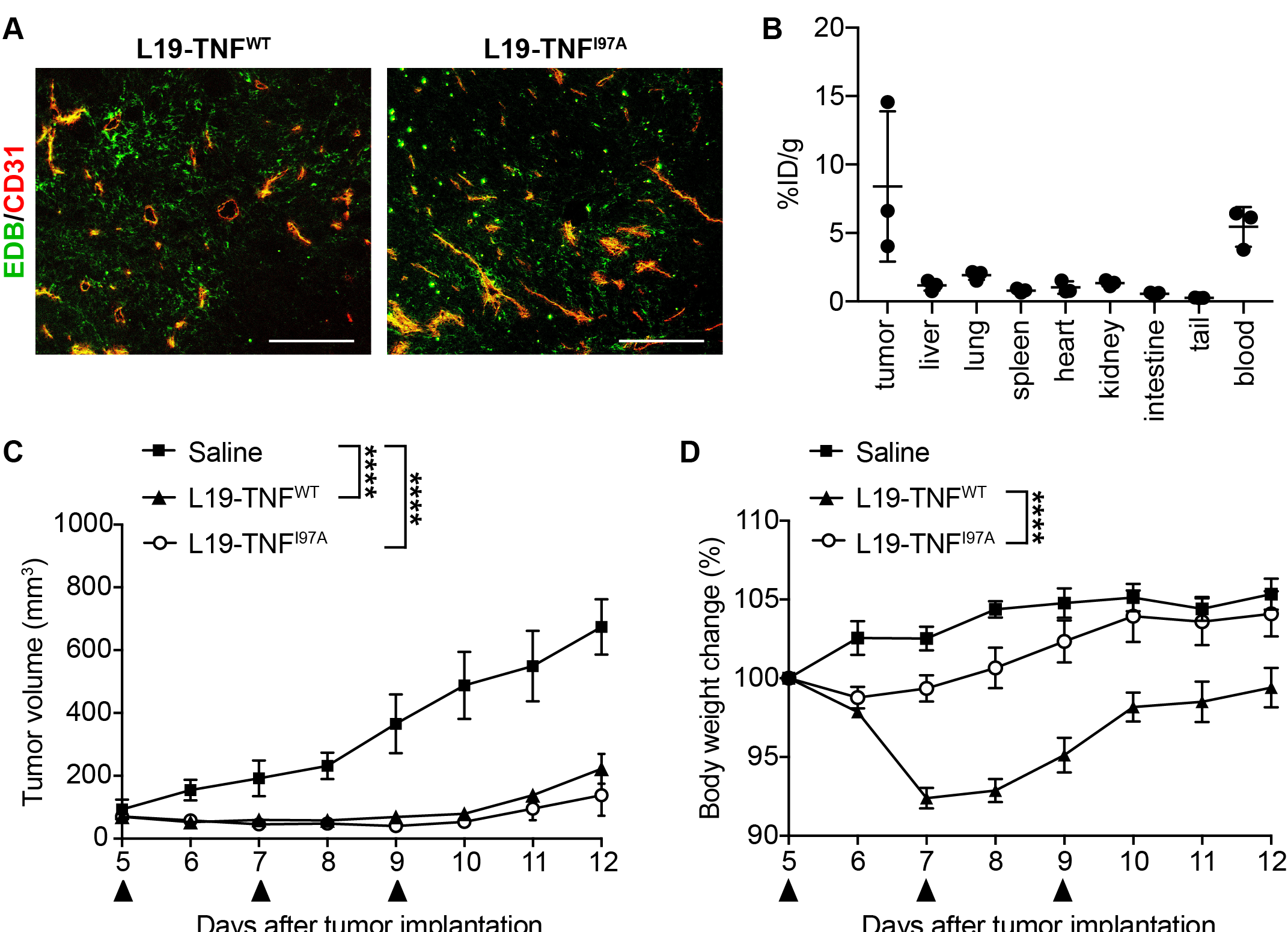
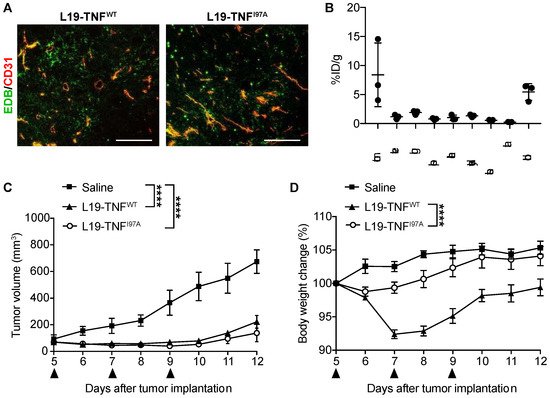
References
- Lee, S.; Margolin, K. Cytokines in cancer immunotherapy. Cancers 2011, 3, 3856–3893.
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22.
- Moudgil, K.D.; Choubey, D. Cytokines in autoimmunity: Role in induction, regulation, and treatment. J. Interferon Cytokine Res. 2011, 31, 695–703.
- Carnemolla, B.; Borsi, L.; Balza, E.; Castellani, P.; Meazza, R.; Berndt, A.; Ferrini, S.; Kosmehl, H.; Neri, D.; Zardi, L. Enhancement of the antitumor properties of interleukin-2 by its targeted delivery to the tumor blood vessel extracellular matrix. Blood 2002, 99, 1659–1665.
- Borsi, L.; Balza, E.; Carnemolla, B.; Sassi, F.; Castellani, P.; Berndt, A.; Kosmehl, H.; Birò, A.; Siri, A.; Orecchia, P.; et al. Selective targeted delivery of TNFα to tumor blood vessels. Blood 2003, 102, 4384–4392.
- Fiers, W. Tumor necrosis factor Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991, 285, 199–212.
- Calcinotto, A.; Grioni, M.; Jachetti, E.; Curnis, F.; Mondino, A.; Parmiani, G.; Corti, A.; Bellone, M. Targeting TNF-α to Neoangiogenic Vessels Enhances Lymphocyte Infiltration in Tumors and Increases the Therapeutic Potential of Immunotherapy. J. Immunol. 2012, 188, 2687–2694.
- Elia, A.R.; Grioni, M.; Basso, V.; Curnis, F.; Freschi, M.; Corti, A.; Mondino, A.; Bellone, M. Targeting tumor vasculature with TNF leads effector t cells to the tumor and enhances therapeutic efficacy of immune checkpoint blockers in combination with adoptive cell therapy. Clin. Cancer Res. 2018, 24, 2171–2181.
- Lejeune, F.J.; Liénard, D.; Matter, M.; Rüegg, C. Efficiency of recombinant human TNF in human cancer therapy. Cancer Immun. 2006, 6, 6.
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors (activated macrophage). Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670.
- Creaven, P.J.; Brenner, D.E.; Cowens, J.W.; Huben, R.P.; Wolf, R.M.; Takita, H.; Arbuck, S.G.; Razack, M.S.; Proefrock, A.D. A phase I clinical trial of recombinant human tumor necrosis factor given daily for five days. Cancer Chemother. Pharmacol. 1989, 23, 186–191.
- Spitaleri, G.; Berardi, R.; Pierantoni, C.; De Pas, T.; Noberasco, C.; Libbra, C.; González-Iglesias, R.; Giovannoni, L.; Tasciotti, A.; Neri, D.; et al. Phase I/II study of the tumour-targeting human monoclonal antibody-cytokine fusion protein L19-TNF in patients with advanced solid tumours. J. Cancer Res. Clin. Oncol. 2013, 139, 447–455.
- Papadia, F.; Basso, V.; Patuzzo, R.; Maurichi, A.; Di Florio, A.; Zardi, L.; Ventura, E.; González-Iglesias, R.; Lovato, V.; Giovannoni, L.; et al. Isolated limb perfusion with the tumor-targeting human monoclonal antibody-cytokine fusion protein L19-TNF plus melphalan and mild hyperthermia in patients with locally advanced extremity melanoma. J. Surg. Oncol. 2013, 107, 173–179.
- Neri, D. Antibody–Cytokine Fusions: Versatile Products for the Modulation of Anticancer Immunity. Cancer Immunol. Res. 2019, 7, 348–354.
- Halin, C.; Rondini, S.; Nilsson, F.; Berndt, A.; Kosmehl, H.; Zardi, L.; Neri, D. Enhancement of the antitumor activity of interleukin-12 by targeted delivery to neovasculature. Nat. Biotechnol. 2002, 20, 264–269.
