Mechanosensitive ion channels mediate the neuronal sensation of mechanical signals such as sound, touch, and pain.
- epithelial cells
- morphogenesis
- mechano-gated ion channels
- calcium ion
1. Introduction
Epithelial cells constitute one of the four general tissue types. Epithelial tissue does not only cover the whole organism as such but also wraps all the visceral organs. Epithelial cells are polarised, i.e., the cortical proteins and organelles are differentially distributed. Tight and septate junctions segregate the apical side from the basal within a typical epithelial sheet, forming a diffusion barrier. In contrast, epithelial cells differ internally within the plane of a tissue sheet, establishing planar cell polarity [1]. Epithelial tissues are meant to undergo a series of morphodynamic and functional changes in the course of development.
Individual epithelial cells communicate chemically or mechanically to promote tissue morphogenesis, remodelling, and pattern formation [2]. Chemical coordination is widespread and long-lived but time-consuming. On the contrary, mechanical communication is almost instantaneous, although spatially limited, between neighbouring cells [3]. It is remarkable how thousands of epithelial cells work in unison to polarise their force-generating types of machinery and remodel their contacts during such tissue-scale changes. The adherens junctions and cytoskeleton mediate mechanical communication. At the centre of adherens junctions, E-cadherin-catenin complexes constitute the mechanical link between neighbours by the Ca 2+ -dependent homotypic trans-binding of two extracellular domains. α-catenin links adherens junctions to the cytoskeleton [4]. Mechanical forces from the actin cytoskeleton prompt the conformational change in α-catenin from its closed to open state, facilitating actin-binding protein vinculin to interact with α-catenin. The link between E-cadherin clusters and the cytoskeleton is reinforced and strengthened in this manner ( Figure 1 a) [5,6][5][6]. Besides adherence junctions, integrin-rich focal adhesion sites at the basal domain of epithelial cells establish mechanical reciprocity between the viscoelasticity of the ECM and the traction force exerted by the cell [7]. Integrins are essential for epithelial polarisation around epidermal wounds in Drosophila, eventually leading to the closure [8].
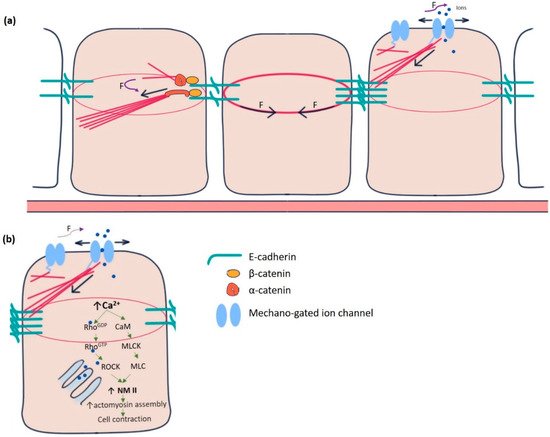
Yet another category of mechanosensitive proteins, called mechano-gated ion channels (MGCs), had long been elusive for functioning in epithelial cell communication ( Figure 1 a). During the last two decades, several researchers gradually pointed out the existence and importance of these channels in the spectrum of model organisms, including Drosophila [9,10,11][9][10][11]. In Drosophila, such channels were initially identified in the sensory neurons, primarily involved in proprioception, nociception, hearing, locomotion, etc. [12,13][12][13]. These channels remain in “open” or “closed” conformational states. The switching between these states is regulated by mechanical force exerted by the plasma membrane or the cytoskeletal proteins [14]. Being in the open state, MGCs exhibit permeability to the ions, such as Ca 2+ , K + , Na + , and Cl − , which act as effector molecules to induce various signalling pathways. For example, Ca 2+ has been shown to promote epithelial tight junction remodelling by activating RhoA in Xenopus embryonic epithelium ( Figure 1 b) [15].
2. Epithelial Cells in Drosophila
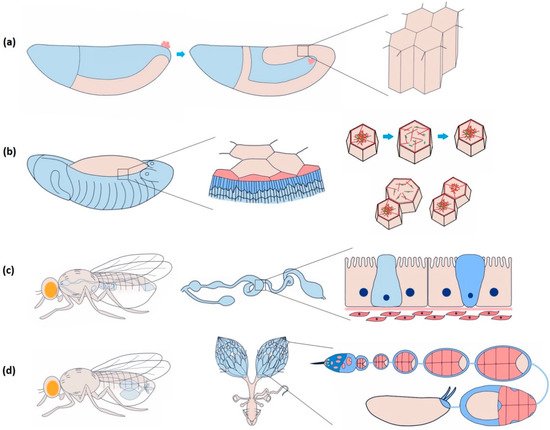
In Drosophila , the epithelial epidermis undergoes a series of spatially defined morphogenetic movements from gastrulation onwards, including tissue invagination, collective cell migration, convergent extension, dorsal closure, tube formation, head involution, etc. [16]. Coordination among the epithelial cells is necessary to ensure tissue integrity for the morphogenetic events to occur seamlessly [17]. Endodermal cell masses from both the ends of the Drosophila embryo collectively migrate along the visceral mesoderm and merge to form the continuous gut epithelium [18]. Yeast ingestion-induced stretching of mature gut epithelium causes yki (yorkie)-mediated proliferation, lacking which the tissue may undergo atrophy [19]. Coordinated asynchronous oscillations of the follicle cells in the Drosophila ovary are essential for egg-chamber elongation [20]. The E-cadherin-based mechanical connection between border and nurse cells is necessary for border cell migration in the egg chamber [21].
3. Calcium Ion in the Epithelium
The importance of calcium signalling in epithelial morphogenesis has been found to be crucial in various model organisms. The convergent extension is defective under the inhibition of calcium signalling in Zebrafish and Xenopus . In contrast, the experimentally induced increase in calcium ion (Ca 2+ ) concentration triggers gastrulation in Echinoidea , neural fold formation in Ambystoma, and egg chamber elongation in Drosophila [22,23,24,25,26][22][23][24][25][26]. Two patterns of Ca 2+ activity have been reported in Drosophila early embryos: (a) Ca 2+ waves that are spontaneous, repetitive, and are often followed by a wave of tissue contraction, and (b) Ca 2+ spikes that arise stochastically in a single cell or a group of few cells and are transient [27]. In the Drosophila wing disc, intracellular Ca 2+ transients act as a signal integrator and decrease over time as the wing disc matures [28,29][28][29]. Increased intracellular Ca 2+ corresponds to intestinal stem cell proliferation in Drosophila by regulating calcineurin and CRTC (CREB-regulated transcriptional co-activator) [30]. In processes like wound healing, Ca 2+ waves help build rapid communication across many cells [31,32][31][32]. Ca 2+ spikes are proven to have a close connection with Wnt signalling, the inhibition of which leads to decreased spike activity and eventually morphogenetic impairment in the developing embryo [33,34,35][33][34][35]. Such waves and spikes temporally coincide and thus hold the potential to regulate various morphogenetic events like dorsal closure, cuticle formation, and head involution [27,35][27][35].
The reciprocity between cell contraction and adherens junction-mediated force transduction to the neighbouring cells contributes to emergent tissue behaviours like folds and furrows formation [36]. Intracellular Ca 2+ has long been a principal regulator of contraction in many cell types, including muscle cells, stromal fibroblasts, and epithelial cells in culture [37,38,39,40][37][38][39][40]. Experiments in Drosophila embryos pointed out the importance of intracellular Ca 2+ to induce contractility in amnioserosa cells during dorsal closure, neural tube closure, and neural plate folding [41,42,43,44][41][42][43][44]. Ventral furrow formation during gastrulation and contraction of amnioserosa cells during dorsal closure revealed the “ratchet” mechanism caused by a pulsatile cortical network of medioapical actomyosin [45]. The contractility in such non-muscle cells is driven by non-muscle myosin II (NM II), primarily regulated by Rho-ROCK signalling [46,47,48][46][47][48]. In the follicle cells of the Drosophila blade, intracellular Ca 2+ seems to control the basal concentration of NM II. Chelating cytosolic Ca 2+ by BAPTA reduces basal NMII. The effect can be reversed by adding ionomycin, driving Ca 2+ influx [26]. Intracellular Ca 2+ can also directly form a complex with tetravalent calmodulin protein, activating myosin light chain kinase (MLCK) that activates the regulatory light chain of NM II ( Figure 1 b) [49,50][49][50].
The amnioserosa is a monolayer of 150–200 autonomously oscillating squamous epithelial cells covering the dorsal opening of developing Drosophila embryos at stages 13–15 ( Figure 2 b) [51,52][51][52]. Inducing rapid Ca 2+ bursts by uncaging intracellular Ca 2+ can trigger amnioserosa cell contraction in single-cell resolution by activating NM II, wherein Ca 2+ is reported to be linked at the position of ROCK (Rho-associated kinase) in the Rho-ROCK pathway [53]. Besides directly phosphorylating the myosin II regulatory light chain (RLC), ROCK also prevents the dephosphorylation of NM II by inhibiting protein phosphatase I (PP I), stabilising the activated NM II [54]. Constitutive activation of MLCK in the entire amnioserosa results in the overall rounding of the cells. Expression in individual cells triggers premature apical constriction [55]. In 3T3 fibroblast cells, ROCK is more centrally localised, whereas MLCK is localised more towards the periphery [50]. This localisation bias has to do with the spatially differential stability of the actomyosin structure within a cell. It could be investigated further to confirm such an argument in epithelial cells.
4. Mechanosensitive Ion Channels in the Epithelium
Not only are highly specialised sensory cells involved in hearing and proprioception, but potentially almost every eukaryotic cell can sense the force from its milieu via the conformational changes of membrane-bound proteins or protein complexes, so-called mechanosensors. These mechanosensors can detect and transduce the external mechanical signal into a cell. Junctional molecules, cytoskeletal proteins, G-protein coupled receptors, and mechanosensitive ion channels (MSCs) constitute a wide range of mechanosensors [56,57][56][57]. MSCs are evolutionarily ancient, pore-forming integral membrane proteins present in literally every living organism from archaea to bacteria to eukaryotes [58]. While in an open state, they allow ions such as Ca 2+ , Na + , K + , and Cl − to flow into and out of cells. The gating behaviour, i.e., the transition from closed to open conformation of MSCs, is regulated either by forces parallel to the plasma membrane (membrane tension model) or by forces applied by the associated cytoskeletal or extracellular matrix proteins (tether model) [14,59][14][59]. Channels are considered mechanically gated if a specific stimulus is immediately followed by the ion flux, at least faster than any other known second messenger and if knockdown of their expression leads to a loss of mechanosensory response [60]. In the following, we will discuss several mechano-gated ion channels, namely, Piezo, transmembrane Channel-like Protein (Tmc), No Mechanoreceptor Potential C (NompC), Transmembrane protein 16 (TMEM16), epithelial sodium channel (DEG/ENaC), and two pore domain K + channel (K2P), in Drosophila epithelial morphogenesis ( Figure 3 ).
Recent studies revealed the involvement of the Piezo channel in epithelial cell homeostasis using MDCK cells in culture. Piezo1 detects and transduces epithelial cell stretch at low-cell-density areas, resulting in cell division [69][61]. Genetic knockdown of Piezo1 hinders homeostatic cell extrusion in developing zebrafish epidermis, leading to the formation of epithelial cell clusters [70][62]. Piezo channels are expressed across the body in many different types of epithelial cells, subject to compressive and shear stresses, for example, vascular endothelial cells, mammary epithelial cells, urinary bladder cells, pancreatic acinar cells, and so on [71][63]. Piezo1 knockout causes embryonic lethality of E14.5 mice due to impaired vasculogenesis [72][64]. Piezo2 has recently been discovered in the human enteroendocrine cell (EEC) population [73][65].
Monovalent cations like sodium ions (Na + ) are implicated in epithelial homeostasis and morphogenesis [113,114][66][67]. Recent studies have found multiple known sodium channels to be mechanically gated. Na v 1.5, a voltage-sensitive sodium channel in the human heart and gut, is activated by membrane stretching. Mutations of this channel disrupt the mechanical sensitivity of gut epithelial cells, resulting in abdominal pain syndrome and irritable bowel syndrome [115][68]. Epithelial sodium channel (ENaC) mediates passive sodium transport at the apical domain of many different epithelial cell types: kidney, lungs, skin, colon, and reproductive tract [116,117,118,119,120][69][70][71][72][73]. ENaC can be activated by shear force across organisms, especially in vascular endothelium, to maintain its tonicity [121][74]. Shear forces can be transduced by the N-glycosylated extracellular domain of ENaC tethering with extracellular matrix (ECM) [122][75]. ENaC directly interacts with spectrin, ankyrin, actin cytoskeleton, and actin-associated proteins [123,124,125][76][77][78]. Cellular responses to hydrostatic pressure differences and membrane stretch depend on such interactions [126][79]. Degenerin (Deg), C. elegans -specific ENaC, gained function mutations resulting in degenerations like swelling, vacuolation, and apoptosis [127][80]. Studying mechanoreceptor currents reveals activation of DEG channels in response to gentle and nociceptive mechanical stimuli [128][81].
Ripped pocket (Rpk) and Pickpocket (Ppk) were identified as two novel ENaC proteins in 1998 in Drosophila . rpk transcripts are abundant in early-stage embryos and adult ovaries, whereas ppk is only expressed in sensory neurons in late-stage embryos [129][82]. It points to the potential functions of r pk during early embryonic development. Rpk localises in patches at the apical surface but not at the junctions of amnioserosa cells. Knockdown of rpk in amnioserosa causes elongation failure of the lateral epidermis. rpk mutant embryos show impaired epitheliogenesis, including defective germband extension, dorsal closure, head involution, and consequent lethality [41]. rpk has recently been implicated in depolarising the membrane potential of anterior epithelial cells during imaginal disc development. Rpk expression in these cells depends on Hedgehog (Hh) signalling. Suppression of rpk leads to a reduction in depolarisation of anterior cells and a disruption in compartmentalisation between anterior and posterior cell populations [130][83].
References
- Campanale, J.P.; Sun, T.Y.; Montell, D.J. Development and dynamics of cell polarity at a glance. J. Cell Sci. 2017, 130, 1201–1207.
- Salm, M.; Pismen, L.M. Chemical and mechanical signaling in epithelial spreading. Phys. Biol. 2012, 9, 026009.
- Janmey, P.A.; Miller, R.T. Mechanisms of mechanical signaling in development and disease. J. Cell Sci. 2011, 124, 9–18.
- Oda, H.; Uemura, T.; Harada, Y.; Iwai, Y.; Takeichi, M. A Drosophila Homolog of Cadherin Associated with Armadillo and Essential for Embryonic Cell-Cell Adhesion. Dev. Biol. 1994, 165, 716–726.
- Ishiyama, N.; Sarpal, R.; Wood, M.N.; Barrick, S.K.; Nishikawa, T.; Hayashi, H.; Kobb, A.B.; Flozak, A.S.; Yemelyanov, A.; Fernandez-Gonzalez, R.; et al. Force-dependent allostery of the α-catenin actin-binding domain controls adherens junction dynamics and functions. Nat. Commun. 2018, 9, 5121.
- Kong, D.; Großhans, J. Planar Cell Polarity and E-Cadherin in Tissue-Scale Shape Changes in Drosophila Embryos. Front. Cell Dev. Biol. 2020, 8, 1710.
- Sun, Z.; Guo, S.S.; Fässler, R. Integrin-mediated mechanotransduction. J. Cell Biol. 2016, 215, 445–456.
- Park, S.-H.; Lee, C.; Lee, J.-H.; Park, J.Y.; Roshandell, M.; Brennan, C.A.; Choe, K.-M. Requirement for and polarized localization of integrin proteins during Drosophila wound closure. MBoC 2018, 29, 2137–2147.
- Maksaev, G.; Haswell, E.S. Expression and characterization of the bacterial mechanosensitive channel MscS in Xenopus laevis oocytes. J. Gen. Physiol. 2011, 138, 641–649.
- Bazopoulou, D.; Tavernarakis, N. Chapter 3—Mechanosensitive Ion Channels in Caenorhabditis elegans. In Current Topics in Membranes; Hamill, O.P., Ed.; Mechanosensitive Ion Channels, Part B; Academic Press: Waltham, MA, USA, 2007; Volume 59, pp. 49–79.
- Hehlert, P.; Zhang, W.; Göpfert, M.C. Drosophila Mechanosensory Transduction. Trends Neurosci. 2021, 44, 323–335.
- Kim, S.E.; Coste, B.; Chadha, A.; Cook, B.; Patapoutian, A. The role of Drosophila Piezo in mechanical nociception. Nature 2012, 483, 209–212.
- Walker, R.G.; Willingham, A.T.; Zuker, C.S. A Drosophila Mechanosensory Transduction Channel. Science 2000, 287, 2229–2234.
- Ranade, S.S.; Syeda, R.; Patapoutian, A. Mechanically Activated Ion Channels. Neuron 2015, 87, 1162–1179.
- Varadarajan, S.; Stephenson, R.E.; Misterovich, E.R.; Wu, J.L.; Erofeev, I.S.; Goryachev, A.B.; Miller, A.L. Mechanosensitive calcium signaling in response to cell shape changes promotes epithelial tight junction remodeling by activating RhoA. bioRxiv 2021.
- Lye, C.M.; Sanson, B. Chapter Five—Tension and Epithelial Morphogenesis in Drosophila Early Embryos. In Current Topics in Developmental Biology; Labouesse, M., Ed.; Forces and Tension in Development; Academic Press: Waltham, MA, USA, 2011; Volume 95, pp. 145–187.
- Köppen, M.; Fernández, B.G.; Carvalho, L.; Jacinto, A.; Heisenberg, C.-P. Coordinated cell-shape changes control epithelial movement in zebrafish and Drosophila. Development 2006, 133, 2671–2681.
- Reuter, R.; Grunewald, B.; Leptin, M. A role for the mesoderm in endodermal migration and morphogenesis in Drosophila. Development 1993, 119, 1135–1145.
- Li, Q.; Nirala, N.K.; Nie, Y.; Chen, H.-J.; Ostroff, G.; Mao, J.; Wang, Q.; Xu, L.; Ip, Y.T. Ingestion of Food Particles Regulates the Mechanosensing Misshapen-Yorkie Pathway in Drosophila Intestinal Growth. Dev. Cell 2018, 45, 433–449.e6.
- Koride, S.; He, L.; Xiong, L.-P.; Lan, G.; Montell, D.J.; Sun, S.X. Mechanochemical regulation of oscillatory follicle cell dynamics in the developing Drosophila egg chamber. MBoC 2014, 25, 3709–3716.
- Cai, D.; Chen, S.-C.; Prasad, M.; He, L.; Wang, X.; Choesmel-Cadamuro, V.; Sawyer, J.K.; Danuser, G.; Montell, D.J. Mechanical feedback through E-cadherin promotes direction sensing during collective cell migration. Cell 2014, 157, 1146–1159.
- Lam, P.Y.; Webb, S.E.; Leclerc, C.; Moreau, M.; Miller, A.L. Inhibition of stored Ca2+ release disrupts convergence-related cell movements in the lateral intermediate mesoderm resulting in abnormal positioning and morphology of the pronephric anlagen in intact zebrafish embryos. Dev. Growth Differ. 2009, 51, 429–442.
- Wallingford, J.B.; Ewald, A.J.; Harland, R.M.; Fraser, S.E. Calcium signaling during convergent extension in Xenopus. Curr. Biol. 2001, 11, 652–661.
- Lane, M.C.; Koehl, M.A.; Wilt, F.; Keller, R. A role for regulated secretion of apical extracellular matrix during epithelial invagination in the sea urchin. Development 1993, 117, 1049–1060.
- Moran, D.; Rice, R.W. Action of papaverine and ionophore A23187 on neurulation. Nature 1976, 261, 497–499.
- He, L.; Wang, X.; Tang, H.L.; Montell, D.J. Tissue elongation requires oscillating contractions of a basal actomyosin network. Nat. Cell Biol. 2010, 12, 1133–1142.
- Markova, O.; Senatore, S.; Lenne, P.-F. Spatiotemporal dynamics of calcium transients during embryogenesis of Drosophila melanogaster. bioRxiv 2019.
- Brodskiy, P.A.; Wu, Q.; Soundarrajan, D.K.; Huizar, F.J.; Chen, J.; Liang, P.; Narciso, C.; Levis, M.K.; Arredondo-Walsh, N.; Chen, D.Z.; et al. Decoding Calcium Signaling Dynamics during Drosophila Wing Disc Development. Biophys. J. 2019, 116, 725–740.
- Balaji, R.; Bielmeier, C.; Harz, H.; Bates, J.; Stadler, C.; Hildebrand, A.; Classen, A.-K. Calcium spikes, waves and oscillations in a large, patterned epithelial tissue. Sci. Rep. 2017, 7, 42786.
- Deng, H.; Gerencser, A.A.; Jasper, H. Signal integration by Ca2+ regulates intestinal stem cell activity. Nature 2015, 528, 212–217.
- Jaffe, L.F. Fast calcium waves. Cell Calcium 2010, 48, 102–113.
- Leybaert, L.; Sanderson, M.J. Intercellular Ca2+ Waves: Mechanisms and Function. Physiol. Rev. 2012, 92, 1359–1392.
- Slusarski, D.C.; Corces, V.G.; Moon, R.T. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature 1997, 390, 410–413.
- Sheldahl, L.C.; Slusarski, D.C.; Pandur, P.; Miller, J.R.; Kühl, M.; Moon, R.T. Dishevelled activates Ca2+ flux, PKC, and CamKII in vertebrate embryos. J. Cell Biol. 2003, 161, 769–777.
- Markova, O.; Lenne, P.-F. Calcium signaling in developing embryos: Focus on the regulation of cell shape changes and collective movements. Semin. Cell Dev. Biol. 2012, 23, 298–307.
- Liang, X.; Gomez, G.A.; Yap, A.S. Current perspectives on cadherin-cytoskeleton interactions and dynamics. CHC 2015, 7, 11–24.
- Kuo, I.Y.; Ehrlich, B.E. Signaling in Muscle Contraction. Cold Spring Harb. Perspect. Biol. 2015, 7, a006023.
- Nobe, K.; Nobe, H.; Obara, K.; Paul, R.J. Preferential role of intracellular Ca2+ stores in regulation of isometric force in NIH 3T3 fibroblast fibres. J. Physiol. 2000, 529, 669–679.
- Lembong, J.; Sabass, B.; Stone, H.A. Calcium oscillations in wounded fibroblast monolayers are spatially regulated through substrate mechanics. Phys. Biol. 2017, 14, 045006.
- Lee, H.C. Calcium in epithelial cell contraction. J. Cell Biol. 1980, 85, 325–336.
- Hunter, G.L.; Crawford, J.M.; Genkins, J.Z.; Kiehart, D.P. Ion channels contribute to the regulation of cell sheet forces during Drosophila dorsal closure. Development 2014, 141, 325–334.
- Lee, H.; Nagele, R.G. Toxic and teratologic effects of verapamil on early chick embryos: Evidence for the involvement of calcium in neural tube closure. Teratology 1986, 33, 203–211.
- Smedley, M.J.; Stanisstreet, M. Calcium and neurulation in mammalian embryos. II. Effects of cytoskeletal inhibitors and calcium antagonists on the neural folds of rat embryos. J. Embryol. Exp. Morphol. 1986, 93, 167–178.
- Suzuki, M.; Sato, M.; Koyama, H.; Hara, Y.; Hayashi, K.; Yasue, N.; Imamura, H.; Fujimori, T.; Nagai, T.; Campbell, R.E.; et al. Distinct intracellular Ca2+ dynamics regulate apical constriction and differentially contribute to neural tube closure. Development 2017, 144, 1307–1316.
- Sutherland, A.; Lesko, A. Pulsed actomyosin contractions in morphogenesis. F1000Research 2020, 9, 142.
- Vicente-Manzanares, M.; Ma, X.; Adelstein, R.S.; Horwitz, A.R. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 2009, 10, 778–790.
- Vasquez, C.G.; Heissler, S.M.; Billington, N.; Sellers, J.R.; Martin, A.C. Drosophila non-muscle myosin II motor activity determines the rate of tissue folding. eLife 2016, 5, e20828.
- Verdier, V.; Chen, G.-C.; Settleman, J. Rho-kinase regulates tissue morphogenesis via non-muscle myosin and LIM-kinase during Drosophila development. BMC Dev. Biol. 2006, 6, 38.
- Hartshorne, D.J.; Ito, M.; Erdo¨di, F. Myosin light chain phosphatase: Subunit composition, interactions and regulation. J. Muscle Res. Cell Motil. 1998, 19, 325.
- Totsukawa, G.; Yamakita, Y.; Yamashiro, S.; Hartshorne, D.J.; Sasaki, Y.; Matsumura, F. Distinct Roles of Rock (Rho-Kinase) and Mlck in Spatial Regulation of Mlc Phosphorylation for Assembly of Stress Fibers and Focal Adhesions in 3t3 Fibroblasts. J. Cell Biol. 2000, 150, 797–806.
- Kiehart, D.P.; Galbraith, C.G.; Edwards, K.A.; Rickoll, W.L.; Montague, R.A. Multiple Forces Contribute to Cell Sheet Morphogenesis for Dorsal Closure in Drosophila. J. Cell Biol. 2000, 149, 471–490.
- Martin, A.C.; Goldstein, B. Apical constriction: Themes and variations on a cellular mechanism driving morphogenesis. Development 2014, 141, 1987–1998.
- Kong, D.; Lv, Z.; Haring, M.; Lin, B.; Wolf, F.; Grosshans, J. In vivo optochemical control of cell contractility at single-cell resolution. EMBO Rep. 2019, 20, e47755.
- Newell-Litwa, K.A.; Horwitz, R.; Lamers, M.L. Non-muscle myosin II in disease: Mechanisms and therapeutic opportunities. Dis. Models Mech. 2015, 8, 1495–1515.
- Homem, C.C.F.; Peifer, M. Diaphanous regulates myosin and adherens junctions to control cell contractility and protrusive behavior during morphogenesis. Development 2008, 135, 1005–1018.
- Chen, Y.; Ju, L.; Rushdi, M.; Ge, C.; Zhu, C. Receptor-mediated cell mechanosensing. Mol. Biol. Cell 2017, 28, 3134–3155.
- Martino, F.; Perestrelo, A.R.; Vinarský, V.; Pagliari, S.; Forte, G. Cellular Mechanotransduction: From Tension to Function. Front. Physiol. 2018, 9, 824.
- Martinac, B.; Kloda, A. Evolutionary origins of mechanosensitive ion channels. Prog. Biophys. Mol. Biol. 2003, 82, 11–24.
- Sharif-Naeini, R. Chapter Three—Contribution of Mechanosensitive Ion Channels to Somatosensation. In Progress in Molecular Biology and Translational Science; Price, T.J., Dussor, G., Eds.; Molecular and Cell Biology of Pain; Academic Press: Waltham, MA, USA, 2015; Volume 131, pp. 53–71.
- Christensen, A.P.; Corey, D.P. TRP channels in mechanosensation: Direct or indirect activation? Nat. Rev. Neurosci. 2007, 8, 510–521.
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 2017, 543, 118–121.
- Eisenhoffer, G.T.; Loftus, P.D.; Yoshigi, M.; Otsuna, H.; Chien, C.-B.; Morcos, P.A.; Rosenblatt, J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484, 546–549.
- Stewart, T.A.; Davis, F.M. Formation and Function of Mammalian Epithelia: Roles for Mechanosensitive PIEZO1 Ion Channels. Front. Cell Dev. Biol. 2019, 7, 260.
- Ranade, S.S.; Qiu, Z.; Woo, S.-H.; Hur, S.S.; Murthy, S.E.; Cahalan, S.M.; Xu, J.; Mathur, J.; Bandell, M.; Coste, B.; et al. Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. USA 2014, 111, 10347–10352.
- Alcaino, C.; Knutson, K.R.; Treichel, A.J.; Yildiz, G.; Strege, P.R.; Linden, D.R.; Li, J.H.; Leiter, A.B.; Szurszewski, J.H.; Farrugia, G.; et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl. Acad. Sci. USA 2018, 115, E7632–E7641.
- Narayanan, V.; Schappell, L.E.; Mayer, C.R.; Duke, A.A.; Armiger, T.J.; Arsenovic, P.T.; Mohan, A.; Dahl, K.N.; Gleghorn, J.P.; Conway, D.E. Osmotic Gradients in Epithelial Acini Increase Mechanical Tension across E-cadherin, Drive Morphogenesis, and Maintain Homeostasis. Curr. Biol. 2020, 30, 624–633.e4.
- George, L.F.; Pradhan, S.J.; Mitchell, D.; Josey, M.; Casey, J.; Belus, M.T.; Fedder, K.N.; Dahal, G.R.; Bates, E.A. Ion Channel Contributions to Wing Development in Drosophila melanogaster. G3 Genes Genomes Genet. 2019, 9, 999–1008.
- Beyder, A.; Rae, J.L.; Bernard, C.; Strege, P.R.; Sachs, F.; Farrugia, G. Mechanosensitivity of Nav1.5, a voltage-sensitive sodium channel. J. Physiol. 2010, 588, 4969–4985.
- Canessa, C.M.; Merillat, A.M.; Rossier, B.C. Membrane topology of the epithelial sodium channel in intact cells. Am. J. Physiol.-Cell Physiol. 1994, 267, C1682–C1690.
- Duc, C.; Farman, N.; Canessa, C.M.; Bonvalet, J.P.; Rossier, B.C. Cell-specific expression of epithelial sodium channel alpha, beta, and gamma subunits in aldosterone-responsive epithelia from the rat: Localization by in situ hybridization and immunocytochemistry. J. Cell Biol. 1994, 127, 1907–1921.
- Hanukoglu, I.; Boggula, V.R.; Vaknine, H.; Sharma, S.; Kleyman, T.; Hanukoglu, A. Expression of epithelial sodium channel (ENaC) and CFTR in the human epidermis and epidermal appendages. Histochem. Cell Biol. 2017, 147, 733–748.
- Hanukoglu, I.; Hanukoglu, A. Epithelial sodium channel (ENaC) family: Phylogeny, structure–function, tissue distribution, and associated inherited diseases. Gene 2016, 579, 95–132.
- Sharma, S.; Kumaran, G.K.; Hanukoglu, I. High-resolution imaging of the actin cytoskeleton and epithelial sodium channel, CFTR, and aquaporin-9 localization in the vas deferens. Mol. Reprod. Dev. 2020, 87, 305–319.
- Wang, S.; Meng, F.; Mohan, S.; Champaneri, B.; Gu, Y. Functional ENaC Channels Expressed in Endothelial Cells: A New Candidate for Mediating Shear Force. Microcirculation 2009, 16, 276–287.
- Knoepp, F.; Ashley, Z.; Barth, D.; Baldin, J.-P.; Jennings, M.; Kazantseva, M.; Saw, E.L.; Katare, R.; de la Rosa, D.A.; Weissmann, N.; et al. Shear force sensing of epithelial Na+ channel (ENaC) relies on N-glycosylated asparagines in the palm and knuckle domains of αENaC. Proc. Natl. Acad. Sci. USA 2020, 117, 717–726.
- Morachevskaya, E.A.; Sudarikova, A.V. Actin dynamics as critical ion channel regulator: ENaC and Piezo in focus. Am. J. Physiol.-Cell Physiol. 2021, 320, C696–C702.
- Ilatovskaya, D.V.; Pavlov, T.S.; Levchenko, V.; Negulyaev, Y.A.; Staruschenko, A. Cortical actin binding protein cortactin mediates ENaC activity via Arp2/3 complex. FASEB J. 2011, 25, 2688–2699.
- Mazzochi, C.; Bubien, J.K.; Smith, P.R.; Benos, D.J. The Carboxyl Terminus of the α-Subunit of the Amiloride-sensitive Epithelial Sodium Channel Binds to F-actin. J. Biol. Chem. 2006, 281, 6528–6538.
- Althaus, M.; Bogdan, R.; Clauss, W.G.; Fronius, M. Mechano-sensitivity of epithelial sodium channels (ENaCs): Laminar shear stress increases ion channel open probability. FASEB J. 2007, 21, 2389–2399.
- Hall, D.H.; Gu, G.; García-Añoveros, J.; Gong, L.; Chalfie, M.; Driscoll, M. Neuropathology of Degenerative Cell Death in Caenorhabditis elegans. J. Neurosci. 1997, 17, 1033–1045.
- Geffeney, S.L.; Goodman, M.B. How we feel: Ion channel partnerships that detect mechanical inputs and give rise to touch and pain perception. Neuron 2012, 74, 609–619.
- Adams, C.M.; Anderson, M.G.; Motto, D.G.; Price, M.P.; Johnson, W.A.; Welsh, M.J. Ripped Pocket and Pickpocket, Novel Drosophila DEG/ENaC Subunits Expressed in Early Development and in Mechanosensory Neurons. J. Cell Biol. 1998, 140, 143–152.
- Emmons-Bell, M.; Hariharan, I.K. Membrane potential regulates Hedgehog signaling in the Drosophila wing disc. EMBO Rep. 2021, 22, e51861.
