Genistein is an isoflavonoid present in high quantities in soy beans. Possessing a wide range of bioactives, it is being studied extensively for its tumoricidal effects. Investigations into mechanisms of the anti-cancer activity have revealed many pathways including induction of cell proliferation, suppression of tyrosine kinases, regulation of Hedgehog-Gli1 signaling, modulation of epigenetic activities, seizing of cell cycle and Akt and MEK signaling pathways, among others via which the cancer cell proliferation can be controlled. Notwithstanding, the observed activities have been time and dose-dependent. In addition, genistein has also shown varying results in women depending on the physiological parameters, such as the early or post-menopausal states.
1. Introduction
Genistein, an isoflavone, is a natural phytoestrogen present in soybeans and native to Southeast Asia. It was first isolated from
Genista tinctoria (L.) in 1899 and named after it, following which it has been mostly identified in the
Trifolium spp., exclusive to the Leguminosae (Fabaceae)
[1].
Several in vitro and in vivo studies have attempted to understand and gain a better insight into the mechanisms underlying the biomedical properties of genistein
[2][3][4]. The isoflavonoid has been analyzed and previously reviewed for its neoplastic potentials. The pathways though which genistein alleviates breast cancer include various grey areas which pertain to the molecular mechanisms of genistein, and preclinical results remain unclear. The identification of the mechanistic action of genistein on breast cancer could help in the development of anti-breast cancer therapy in cases where there are no targeted therapies known or available. Further research into the mechanistic action of genistein could lead to the development of a potential plant-based cancer drug with minimal deleterious effects, along with overcoming drug resistance and repression of reoccurrence of cancers. Such a development of genistein in chemotherapy may be a powerful tool in personalized medicine.
2. Chemistry of Genistein
2.1. Structure
In plants, the synthesis of genistein starts from a flavanone, naringenin, by the isoflavone synthase enzyme due to ring migration
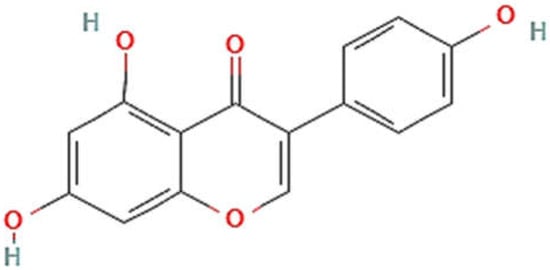
[1][3]. The structure of genistein (chemically, 4′,5,7-trihydroxyisoflavone (C15H10O5)) and estradiol have been observed to be similar
[5]; hence, genistein has estrogenic activity and is a good example of a phyto-estrogenic substance. Its nucleus is made up of two arenes (A and B) coupled to another carbon ring (C). It has a limited water solubility and a preference for polar solvents such as acetone and ethanol. It has a C2-C3 double bond in its basic carbon skeleton, as well as an oxo-group in the C ring at the C4 position along with 3 hydroxyl groups at the C 4′, 5, and 7 locations of rings A and B
[4]. The structure of genistein is illustrated in
Figure 1.
2.2. Synthesis of Genistein
Baker was the first to synthesize genistein organically in 1928
[6] using deoxybenzoin as a substrate. The cyclization of ketones was used as a chemical method of genistein synthesis in an oven
[7]. Its synthesis from 2,4,6-trihydroxyphenyl ethenone with the two hydroxyl substituents in the triol as methoxymethyl ester has been attempted using a technique that begins with ketone production, followed by closing of the ring structure and a Suzuki coupling reaction with palladium acetate and polyethylene glycol
[8]. Treatment of trihydroxybenzoin, derived by acylation of phloroglucinol substituted with phenyl acetonitrile using hydrochloric acid and zinc chloride with catalyst dry ether, is a more contemporary technique to genistein production
[9]. Biotechnological synthesis was accomplished by converting (2S)-naringen to genistein under NAD(P)H and oxygen-dependent states and adding cytochrome P-450 to soybean cell cultures
[10]. Employing genetically modified
Saccharomyces cerevisiae cells containing the isoflavone synthase gene obtained from
Glycyrrhyza echinata, a metabolic approach along with engineering tools was set up as genistein synthesis
[11]. In
Nicotiana tabacum leaves transformed with IFS, genistein was created via acting on the phenylpropanoid pathway; however, ultraviolet ray treatment also increased genistein assembly
[12]. Biological genistein synthesis from p-coumaric acid or naringenin was attempted utilizing
Escherichia coli as a biotransformation host using Os4CL, PeCHS, RcIFS, and OsCPR for production
[13].
2.3. Synthesis of Genistein Derivates or Analogues
Synthesis of analogues of genistein was achieved by the Ferrier rearrangement of compounds yielding 2,3-unsaturated bromo-alkyl-glycosides, which were then epoxidated with meta-chloroperoxybenzoic acid before coupling with genistein
[14]. For the manufacture of genistein derivatives, new glycosylation and glycoconjugation chemical techniques have been devised
[15]. A novel three-step synthesis from genistein of a water-soluble compound was also attempted, in which base-catalyzed reaction of genistein was hydrolyzed to obtain the target compound
[16].
2.4. Bioavailability and Metabolism of Genistein
The amount of a component that is absorbed in the body is known as bioavailability. It is critical to research a chemical’s bioavailability in order to determine how effective it is on the body. Poor water solubility of genistein is a limitation to overcome for its bioavailability after oral administration, for which water-soluble derivatives of genistein were synthesized
[17]. Because of its low molecular weight (270 kDa) and lipophilic characteristics, genistein is quickly absorbed in the intestine in both rodents and humans
[18]. A very low half-life of approximately 46 h was observed in vivo following oral administration
[19]. Glucuronidation and sulfation are major pathways of metabolism of genistein with the production of metabolites
[18]. Once consumed, genistein is converted into genistein glucuronide and sulphate in the intestine, which along with genistein circulate through veins with the assistance of multidrug resistance-associated protein 3 transporters with a 100% absorption ratio
[20]. The metabolites are excreted through bile or through kidneys. In humans, micromolar levels of genistein in blood can be found through prolonged dietary exposure
[20][21]. Metabolomic studies may be required in order to assess the intracellular concentrations of genistein at which modulation of a range of targets occur and hence, careful attention is required towards the dose-dependent behavior of genistein, as well as the pertaining molecular intricacy
[22][23]. One main limitation with genistein being a natural compound is its low water solubility, which may need to be modified with respect to its chemical structure in order to increase solubility and have higher bioavailability
[24]. Furthermore, studies may need to be performed on identifying the purified individual versus mixture of isoflavones present in breast cancer. However, studies observing the pharmacological and biomedical activity of unbound genistein in comparison with its metabolic products are less. Hence, it is important to evaluate free, unbound genistein concentration in blood. Being bitter in taste, genistein requires different formulations in order to overcome the taste, as well as the limitation of bioavailability.
3. Genistein and Cancer
Genistein has demonstrated a plethora of biomedical effects, such as anti-oxidation, anti-proliferation, and tumoricidal activities
[25]. More importantly, in vivo, in vitro, as well as in silico research into its anti-cancer properties have pointed towards a pivotal role played by genistein as an anti-tumoricidal molecule in varied types of cancer
[26]. Two very important reasons for the extensive research conducted on genistein over the past decade are the evidence of lower risk of diseases in association with its administration and to look for pharmacologic drugs that affect with growth factor signaling pathways in cells.
Numerous previous studies have reported arrest of cell-division cycle and apoptosis in multiple cancer cell lines in in vitro studies, as well as demonstration of the same in vivo
[4][25]. When researchers looked at the consequences of genistein on cell cycle progression in prostate cancer cell lines, they discovered that it stopped cell-division cycles in the G2/M phases due to the downregulation of cyclin B expression, leading to the conclusion that it could be a potent regulator of cyclin B with potential applications in cancer prevention
[27]. In a study of the pleiotropic molecular effects of genistein on head cancer cells, researchers discovered that genistein causes molecular alterations in the cancer cells that impede cell development and induce apoptosis. In a series of tests, the same researchers discovered that genistein halted progression through the cell cycle and death in a head cancer cell line through regulating p21WAF1 and Bax, as well as repressing cyclin B1 and Bcl-2. They further confirmed that genistein reduces metaphase chromosomal spread and hampers nuclear translocation of human telomerase reverse transcriptase without impacting telomerase activity via downregulating cerbB-2
[28]. Some recently discovered mechanisms employed by genistein in various cancer models to bring about anti-cancer effect are summarized in
Table 1.
Table 1.
Some recently discovered anti-cancer mechanisms of genistein.
| Effect |
Mechanism |
Cancer Model |
Reference |
| Evasion of Apoptosis |
ER-stress |
HL-60 |
[29] |
| ↑ROS |
Mia-PaCa2 and PANC-1 |
[30] |
| Cell cycle arrest |
G0/G1arrest |
Mia-PaCa2 and PANC-1 |
[30] |
| Mitotic arrest, ↓PlK1 |
TP53-mutated A460 cancer cells |
[31] |
| Anti-metastatic |
↓DMBA-induced metastatic transition |
Mouse model |
[32] |
| Anti-proliferative |
↑p-ERK |
Mouse model |
[33] |
| ↑BDNF |
| ↓AChE |
| ↓mTOR |
Hen model |
[34] |
| ↓p70S6K1 |
| ↓4E-BP1 |
| ↓Bcl-2 |
| ↑Nrf2 |
| ↑HO-1 |
| ↑Bax |
| ↓HDACs |
HeLa cells |
[35] |
ER—Estrogen Receptor; ROS—Reactive Oxygen Species; PlK1—Polo-Like Kinase 1; DMBA—7,12-Dimethylbenz[a]anthracene; p-ERK—Phosphorylated Extracellular Signal-Regulated Kinase; BDNF—Brain-Derived Neurotrophic Factor; AChE—Acetylcholinesterase; mTOR—Mammalian target of rapamycin; p70S6K1—Ribosomal protein S6 kinase β 1; 4E-BP1—Eukaryotic translation initiation factor 4E-binding protein 1; Bcl-2—BCL2 apoptosis regulator gene; Nrf2—Nuclear factor erythroid 2-related factor 2; HO-1—Heme Oxygenase 1; Bax—BCL2 Associated X, Apoptosis Regulator gene; HDACs—Histone Deacetylases.