As Mitochondria are semi-autonomous intracellular double membrane-bound oran essential organelles, which include an outer membrane, a highly folded inner membrane (crista), a matrix space surrounded by the inner membrane, and an inter-membrane space between the inner and outer membranes. Usually, a cell has hundreds or thousands of in nucleated eukaryotic cells, mitochondria, which can occupy up to 25% of the cellular cytoplasm. Mitochondria are a convergence point for glucose, glutamine, and lipid play a central role in energy metabolism. The primary function of mitochondria is to support the TCA cycle and aerobic respiration by oxidative phosphorylation, generating ATP through the mitochondrial respiratory chain to fulfill the energy needs for cell survival. One unique feature of mitochondria is that they possess their own supercoiled, double-stranded circular genetic material called mitochondrial DNA (mtDNA) that encodes rRNAs, tRNAs, and proteins essential for electron transport and oxidative phosphorylation, as well as their own genetic repair mechanisms, maintenance of redox balance, and regulation of apoptosis. Mitochondrial biogenesis requires the coordinated expression of both mtDNA- and nuclear DNA-encoded genes. Thirteen proteins are encoded by mtDNA, while approximately 1000 mitochondrial proteins are encoded by the nuclear genome, translated in the cytoplasm and transported into the mitochondria by a specific transport system. These two pools of proteins are required to maintain mitodysfunction, either due to the TCA cycle enzyme defects, mitochondria as a cellular power hub and a signaling nexus that are essential for normal cell function. Defects in many of the mitochondrial components are causal for a multitude of cellular diseases. Of note, the reprogramming of cellular metabolism and the aberrant redox status have been heralded as major emerging hallmarks of neoplastic transformation. Overall,l DNA genetic mutations, defective mitochondrial dysfunction caused by mtDNA mutations, malfunctioned TCA cycle enzymes, electron respiratoryelectron transport chain leakage and subsequent , oxidative stress, and/or aberrant oncogenice and tumor suppressor signaling is known to alter cellular metabolic pathways, disrupt redox balance, and cause resistance to apoptosis and therapies that significantly contribute to the development of multiple types of , has been observed in a wide spectrum of human cancers. In the following sections, we will present current knowledge on these aspects ofThis review summarizes mitochondrial dysfunction pertaining to the pathologies of various forms ofinduced by these alterations that promote human malignanciecancers.
- mitochondria
- dysfunction
- cancers
- TCA cycle
- electron transport chain
- oxidative phosphorylation
- oncogene
- tumor suppressor
1. Mitochondrial TCA Cycle and Human Cancer
1. Introduction
Glucose represents a key component of the daily diet and can be actively transported into cells and converted into pyruvate by glycolysis in the cytosol. When oxygen is limited, pyruvate is converted into lactate by lactate dehydrogenase (LDH). In the presence of oxygen, pyruvate can be transported into mitochondria and decarboxylated to produce acetyl CoA by the pyruvate dehydrogenase complex (PDH) in the mitochondria. The oxidation of acetyl CoA is achieved by the tricarboxylic acid (TCA) cycle, which involves a set of metabolic reactions catalyzed by specific enzymes including citrate synthase, aconitase, isocitrate dehydrogenase (IDH), α-ketoglutarate (α-KG) dehydrogenase complex, succinyl-CoA synthase, succinate dehydrogenase (SDH), fumarate hydratase (FH), and malate dehydrogenase in the mitochondria to generate CO
2. Mitochondrial TCA Cycle and Human Cancer
2
2
. The TCA cycle represents the final converging route for the oxidation of lipids, carbohydrates, and amino acids [3]. Recent studies have shown that mutations in several enzymes involved in the TCA cycle can induce various human cancers.
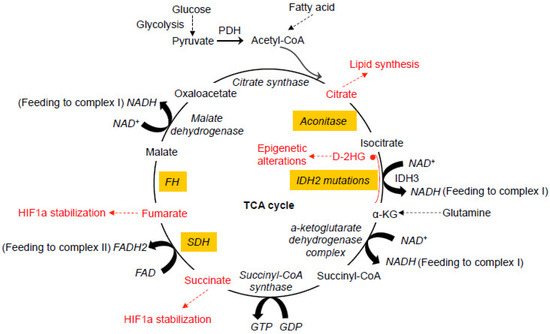
Figure 1.
23. Mitochondrial DNA Mutations and Respiratory Chain Dysfunction in Cancer
2.1. Mitochondrial DNA Mutations in Cancer
3.1. Mitochondrial DNA Mutations in Cancer
Mutations in mtDNA have been found in a spectrum of human cancers [4][5][6] . They can occur at the D-loop region, the protein-coding region, the rRNA genes, and the tRNA genes [7][8][9]. The most common mtDNA alterations in cancers are point mutations and reduced copy numbers, but frame-shifting insertions and large-scale deletions have also been identified [7]. The D-loop region of mtDNA is regarded as the most frequent site of somatic mutations in many cancers. Because mtDNA replication starts at the D-loop, mutations in the D-loop region can affect mtDNA copy number in cancers. In a study investigating mtDNA mutations in 31 gastric cancer patients, 51.6% of mtDNA mutations occurred in the D-loop region, 22.6% in the protein-coding region, and 4% in tRNA genes [10]. Through sequencing analysis of the entire mtDNA from 58 breast cancer samples with paired non-tumorous breast tissues, researchers identified that 52.5% of mtDNA mutations were located in the D-loop region, 37.5% in protein-coding region, and 5% each in the rRNA and tRNA genes. These somatic mutations are thought to cause mitochondrial dysfunction by altering the functions of 12S rRNA (T1499C), 16S rRNA (G1913A), tRNA
Trp
Cys (G5809A), as well as ND2 (G5112A), ND4L (G10599A), ND5 (A13878G) of complex I, cytochrome b (T15416C) of complex III, and COI (G6384A, G6768A) and COIII (G9412A, G9774A, A9901C) of complex IV [9]. In hepatocellular carcinoma, somatic mtDNA mutations that affect the functions of tRNA
Val
Ala (G5650A), ND1 (G3842A), ND4 (11032delA, A11708G), ND5 (12418insA), COI (T6787C), COII (G7976A), and COIII (A9263G, G9267A) were also observed [8]. By sequencing the mitochondrial genomes of 384 prostate cancer patients, researchers found that 15.4% of the tumors harbor mutations in the non-coding control region of mitochondrial genome [11], while ND5 was the most frequently mutated gene in the protein-coding region [11]. Furthermore, sequence analyses of 226 paired tumor and normal tissue samples in five cancers by The Cancer Genome Atlas (TCGA) revealed that the frequencies of deleterious tumor-specific somatic mtDNA mutations are 63% in rectal adenocarcinomas, 53% in colon adenocarcinomas, 36% on ovarian serous cyst adenocarcinomas, 30% in acute myeloid leukemia, and 13% in glioblastoma, highlighting the prevalence of mtDNA mutations in diverse types of cancers. These mtDNA mutations are predicted to impact the functions of encoded proteins that confer a selective advantage in oncogenesis [12]. Some mutation hotspots have been observed in several cancers. The 12418insA mutation, with an adenosine nucleotide insertion in a poly-A sequence at nucleotide position 12,418–12,425 of mtDNA, causes a frame-shift and premature termination of the ND5 gene and results in a truncated polypeptide. This mutation was observed in colorectal cancer, breast cancer, gastric cancer, and hepatocellular cancer and is correlated with defective mitochondrial respiration, higher levels of lactate production, and increased tumorigenesis [8][9][10][13]. By using a cybrid cell model, a heteroplasmic 12418insA mutation was shown to increase ROS generation and decrease oxidative phosphorylation in human cancer cells, as well as promote tumor growth in nude mice [14], suggesting that this hotspot mutation plays a critical role in tumorigenesis. Of note, the G-to-A and T-to-C transitions, which are typical changes that occur upon exposure to free radicals, are rather common, suggesting that these mutations are associated with oxidative stress. In a mtDNA analysis of colon cancer patients, it was found that 70% of the identified mtDNA mutations were T-to-C and G-to-A replacements [15]. Similarly, the G-to-A replacements in the ATP6 gene at positions 8557, 8697, and 8854 of mtDNA were found in breast cancer patients [16]. The accumulation of mtDNA mutations was found to correlate with the degree of malignancy [17]. Furthermore, mutations in mtDNA exacerbate ROS production and oxidative stress [18] to promote more mtDNA mutations, which, in turn, create a vicious cycle critical for tumorigenesis.
2.2. The Complexity in the Roles of mtDNA Mutations in Tumorigenesis
3.2. The Complexity in the Roles of mtDNA Mutations in Tumorigenesis
Although the majority of studies suggested a positive role of mtDNA mutations in neoplastic progression, it should be noted that a clear link between mtDNA mutations and tumorigenesis has yet to be established, in part owing to the hurdle in our ability to directly manipulate mtDNA and the complexity of mutational landscape obtained from different experimental models and tumor samples. It thus remains a gap in knowledge on mtDNA mutations and their biological significance in tumor formation, progression, and metastasis. In some preclinical cellular and mouse xenograft models, depletion of mtDNA in tumor cells was shown to decrease tumorigenic phenotypes or potential [19], while the acquisition of normal mtDNA, e.g., via horizontal transfer of the whole mtDNA from surrounding cells, could restore functional mitochondrial respiration and tumorigenic potential of mtDNA-depleted cancer cells [20][21][22]. These studies indicate that mitochondrial respiration or oxidative phosphorylation may be required for tumorigenic potential under certain conditions. Some mtDNA mutations can be found in both normal and cancer tissues. The deletion of mtDNA at 4977-bp has been implicated in the contribution to malignant transformation in breast cancer patients; however, it was also observed in the benign tissues and surrounding normal tissues [23]. Furthermore, analysis of random point mutations in the mtDNA of cancerous and healthy tissues from 21 colorectal cancer patients revealed that the frequency of mtDNA mutations decreased in colorectal tumors relative to the adjacent healthy tissues [24], suggesting a mutation selection during tumor development and that not all mtDNA mutations contribute to cancer development—some may suppress tumor growth [24]. Using different transmitochondrial cytoplasmic cybrid cell models harboring a panel of pathogenic mtDNA mutations that disrupt the respiratory chain, studies showed that defects in the respiratory chain could either promote or inhibit cell death, depending on the specific alterations in the electron flow and cytosolic cues (e.g., ER stress) [25].
Although the majority of studies suggested a positive role of mtDNA mutations in neoplastic progression, it should be noted that a clear link between mtDNA mutations and tumorigenesis has yet to be established, in part owing to the hurdle in our ability to directly manipulate mtDNA and the complexity of mutational landscape obtained from different experimental models and tumor samples. It thus remains a gap in knowledge on mtDNA mutations and their biological significance in tumor formation, progression, and metastasis. In some preclinical cellular and mouse xenograft models, depletion of mtDNA in tumor cells was shown to decrease tumorigenic phenotypes or potential [117], while the acquisition of normal mtDNA, e.g., via horizontal transfer of the whole mtDNA from surrounding cells, could restore functional mitochondrial respiration and tumorigenic potential of mtDNA-depleted cancer cells [118,119,120]. These studies indicate that mitochondrial respiration or oxidative phosphorylation may be required for tumorigenic potential under certain conditions. Some mtDNA mutations can be found in both normal and cancer tissues. The deletion of mtDNA at 4977-bp has been implicated in the contribution to malignant transformation in breast cancer patients; however, it was also observed in the benign tissues and surrounding normal tissues [121]. Furthermore, analysis of random point mutations in the mtDNA of cancerous and healthy tissues from 21 colorectal cancer patients revealed that the frequency of mtDNA mutations decreased in colorectal tumors relative to the adjacent healthy tissues [122], suggesting a mutation selection during tumor development and that not all mtDNA mutations contribute to cancer development—some may suppress tumor growth [122]. Using different transmitochondrial cytoplasmic cybrid cell models harboring a panel of pathogenic mtDNA mutations that disrupt the respiratory chain, studies showed that defects in the respiratory chain could either promote or inhibit cell death, depending on the specific alterations in the electron flow and cytosolic cues (e.g., ER stress) [123].Oncocytoma is a rare, predominantly benign neoplasm characterized by the dramatic accumulation of defective mitochondria that have mtDNA mutations in the control region and protein-encoding region that induce respiration chain defects [26]. The common mtDNA gene mutations in oncocytomas include
Oncocytoma is a rare, predominantly benign neoplasm characterized by the dramatic accumulation of defective mitochondria that have mtDNA mutations in the control region and protein-encoding region that induce respiration chain defects [124]. The common mtDNA gene mutations in oncocytomas includeCOI, COII, COIII, ND4, ND5,
andCYTB. It has been a puzzle about what restricts oncocytomas to remain a benign disease despite such a spectrum of mutations. A recent study suggests that these genetic defects in mitochondrial respiration block Golgi to lysosome trafficking and autophagy, and activate AMPK and p53, thus limiting tumor growth to only a benign state [27]. The type 2 oncocytomas are closely related to the eosinophilic subtype of chromophobe renal cell carcinoma (ChRCC), as they share similarities in the mutational landscape and transcriptome profile, with ChRCC having acquired additional driver mutations in p53 and PTEN and further genetic instability [27]. This study indicates that the impaired mitochondrial function may be a barrier to tumorigenesis by activating p53 in oncocytomas, while subsequent alterations of p53 and other nuclear genes lead to malignant ChRCC.
. It has been a puzzle about what restricts oncocytomas to remain a benign disease despite such a spectrum of mutations. A recent study suggests that these genetic defects in mitochondrial respiration block Golgi to lysosome trafficking and autophagy, and activate AMPK and p53, thus limiting tumor growth to only a benign state [125]. The type 2 oncocytomas are closely related to the eosinophilic subtype of chromophobe renal cell carcinoma (ChRCC), as they share similarities in the mutational landscape and transcriptome profile, with ChRCC having acquired additional driver mutations in p53 and PTEN and further genetic instability [125]. This study indicates that the impaired mitochondrial function may be a barrier to tumorigenesis by activating p53 in oncocytomas, while subsequent alterations of p53 and other nuclear genes lead to malignant ChRCC.In brief, although the majority of evidence supports a role of mtDNA mutations in tumorigenesis and malignant progression, the battle to determine whether an mtDNA mutation is a driver mutation for cancer initiation and progression or merely a passenger mutation that does not determine the development of cancer is predicted to continue. The biological impact of a given mtDNA mutation may vary, depending on the nature of the mutation, the proportion of the mutation in the cell, the effects of the mutation to the respiratory chain, and the interplay of the mutant mtDNA-directed events with cytoplasmic signal pathways. Innovation in mtDNA manipulation in well-defined model systems is essential for clarifying the complexity.
In brief, although the majority of evidence supports a role of mtDNA mutations in tumorigenesis and malignant progression, the battle to determine whether an mtDNA mutation is a driver mutation for cancer initiation and progression or merely a passenger mutation that does not determine the development of cancer is predicted to continue. The biological impact of a given mtDNA mutation may vary, depending on the nature of the mutation, the proportion of the mutation in the cell, the effects of the mutation to the respiratory chain, and the interplay of the mutant mtDNA-directed events with cytoplasmic signal pathways. Innovation in mtDNA manipulation in well-defined model systems is essential for clarifying the complexity.34. Mitochondrial Oxidative Stress and Cancer
The process of mitochondrial oxidative phosphorylation consumes more than 90% of oxygen. Although the most portion of oxygen is fully reduced to water by cytochrome c oxidase, 1–2% is incompletely reduced to superoxide radicals mainly in complex I and III of the respiratory chain [28][29][30][31]. Superoxide in the mitochondrial matrix can be rapidly dismuted by mitochondrial superoxide dismutase 2 (SOD2) yielding H
The process of mitochondrial oxidative phosphorylation consumes more than 90% of oxygen. Although the most portion of oxygen is fully reduced to water by cytochrome c oxidase, 1–2% is incompletely reduced to superoxide radicals mainly in complex I and III of the respiratory chain [126,127,128,129]. Superoxide in the mitochondrial matrix can be rapidly dismuted by mitochondrial superoxide dismutase 2 (SOD2) yielding H2
O2, which can be released into the intermembrane space and cytosol leading to the generation of cytoplasmic ROS. In the presence of a reduced transition metal, H
, which can be released into the intermembrane space and cytosol leading to the generation of cytoplasmic ROS (Figure 2). In the presence of a reduced transition metal, H2
O2 can be converted into the highly reactive hydroxyl radical OH· [32][33][34][35]. The unpaired electrons are highly reactive, producing chemical modifications that damage proteins, lipids, and nucleotides [36]. Under physiological conditions, free radicals and their derivatives exist in living tissues at low but measurable concentrations, which are determined by the balance between the rate of radical production and the rate of clearance. Free radicals can be eliminated by the reducing equivalent GSH, an abundant cellular thiol and a major determinant of cellular redox equilibrium [37]. Other antioxidant enzymes such as glutathione peroxidase (GPX), glutathione reductase (GR), peroxiredoxin (PRX), thioredoxin, and thioredoxin reductase also play important roles in the clearance of free radicals and the maintenance of redox homeostasis [38].
can be converted into the highly reactive hydroxyl radical OH· [130,131,132,133]. The unpaired electrons are highly reactive, producing chemical modifications that damage proteins, lipids, and nucleotides [134]. Under physiological conditions, free radicals and their derivatives exist in living tissues at low but measurable concentrations, which are determined by the balance between the rate of radical production and the rate of clearance. Free radicals can be eliminated by the reducing equivalent GSH, an abundant cellular thiol and a major determinant of cellular redox equilibrium [135]. Other antioxidant enzymes such as glutathione peroxidase (GPX), glutathione reductase (GR), peroxiredoxin (PRX), thioredoxin, and thioredoxin reductase also play important roles in the clearance of free radicals and the maintenance of redox homeostasis [136]. Mitochondrial respiratory chain dysfunction induced by mtDNA mutations can cause an increase in electron leakage, resulting in an elevation of free radical generation as widely observed in cancer cells [93,137]. In most cells, mitochondrial complex I and complex III, as well as membrane-bound NADPH oxidases (NOXs) are the main sources of cellular ROS [104,108,138,139,140,141]. Interestingly, mitochondrial respiratory chain dysfunction can induce NOX activation [77,142], suggesting that NOX can be activated in response to mitochondrial dysfunction, contributing to heightened levels of cellular ROS. The escalated ROS generation in cancer cells serves as a messenger to stimulate cell proliferation and as an endogenous source of DNA-damaging agents to promote genetic instability and the development of cancer (Figure 2) [143]. DNA contains a large number of ROS-reactive sites [144]. Oxidative damages to DNA may be in the form of base modifications (such as 8-oxoguanine), abasic sites, strand breaks, or various other types of lesions [145]. Of note, ROS production in mitochondria renders mtDNA more susceptible to damage and mutagenesis than the nuclear genome. This is because mtDNA lacks histone protection, has limited DNA damage repair capacity, and is in close proximity to the electron transport chain. As mitochondrial transcription is polycistronic, deletion or insertion of a nucleotide may readily cause the downstream codon frameshifting, resulting in an inability to encode functional products [146]. It was reported that the rate of mtDNA mutation is 10–20 times greater than that of nuclear DNA [147]. Thus, ROS-induced mtDNA mutations and the high susceptibility of mtDNA to mutations play key roles in many diseases, including cancer, aging, and neurodegenerative diseases.Mitochondrial respiratory chain dysfunction induced by mtDNA mutations can cause an increase in electron leakage, resulting in an elevation of free radical generation as widely observed in cancer cells [39]. In most cells, mitochondrial complex I and complex III, as well as membrane-bound NADPH oxidases (NOXs) are the main sources of cellular ROS [40][41][42][43]. Interestingly, mitochondrial respiratory chain dysfunction can induce NOX activation [44], suggesting that NOX can be activated in response to mitochondrial dysfunction, contributing to heightened levels of cellular ROS. The escalated ROS generation in cancer cells serves as a messenger to stimulate cell proliferation and as an endogenous source of DNA-damaging agents to promote genetic instability and the development of cancer [45]. DNA contains a large number of ROS-reactive sites [46]. Oxidative damages to DNA may be in the form of base modifications (such as 8-oxoguanine), abasic sites, strand breaks, or various other types of lesions [47]. Of note, ROS production in mitochondria renders mtDNA more susceptible to damage and mutagenesis than the nuclear genome. This is because mtDNA lacks histone protection, has limited DNA damage repair capacity, and is in close proximity to the electron transport chain. As mitochondrial transcription is polycistronic, deletion or insertion of a nucleotide may readily cause the downstream codon frameshifting, resulting in an inability to encode functional products [48]. It was reported that the rate of mtDNA mutation is 10–20 times greater than that of nuclear DNA [49]. Thus, ROS-induced mtDNA mutations and the high susceptibility of mtDNA to mutations play key roles in many diseases, including cancer, aging, and neurodegenerative diseases.
Notably, some key enzymes for energy metabolism can be directly targeted by free radicals. The Fe-S clusters are essential components of the redox-active enzymes within both the TCA cycle and the respiratory chain [148]. Excess superoxide and hydroxyl radicals are capable of reacting with the Fe-S clusters in NADH dehydrogenase, SDH, aconitase, and other enzymes, resulting in their inactivation and subsequent inhibition of production of biological intermediates and energy [149]. NADH dehydrogenase is one of the key enzymes involved in the oxidative phosphorylation, and aconitase is the first rate-limiting enzyme converting citrate into isocitrate in the TCA cycle generating high energy reducing equivalent NADH. Aconitase has been found to be significantly reduced in tumor cells [150]. The impaired activity of aconitase causes a truncation to the TCA cycle, leading to the exportation of considerably large amounts of citrate [151], an essential precursor for the de novo biosynthesis of cholesterol and fatty acid.Notably, some key enzymes for energy metabolism can be directly targeted by free radicals. The Fe-S clusters are essential components of the redox-active enzymes within both the TCA cycle and the respiratory chain [50]. Excess superoxide and hydroxyl radicals are capable of reacting with the Fe-S clusters in NADH dehydrogenase, SDH, aconitase, and other enzymes, resulting in their inactivation and subsequent inhibition of production of biological intermediates and energy [51]. NADH dehydrogenase is one of the key enzymes involved in the oxidative phosphorylation, and aconitase is the first rate-limiting enzyme converting citrate into isocitrate in the TCA cycle generating high energy reducing equivalent NADH. Aconitase has been found to be significantly reduced in tumor cells [52]. The impaired activity of aconitase causes a truncation to the TCA cycle, leading to the exportation of considerably large amounts of citrate [53], an essential precursor for the de novo biosynthesis of cholesterol and fatty acid.
SOD1 is a major superoxide-scavenging enzyme catalyzing the conversion of O2− to H2O2 in multiple cellular locations, including the cytoplasm, mitochondrial intermembrane space, nucleus, and lysosomes. More than 30% of mice deficient in SOD1 developed liver tumors by 20 months of age [47]. Extensive oxidative damage, including increased DNA damage, protein oxidation, and lipid peroxidation, and decreased cytosolic aconitase activity, were observed in the derived liver cancer cells. Abnormal mitochondria with disorganized cristae and reduced size were also seen in these cancer cells. As a family member of SOD1, SOD2 catalyzes the dismutation of superoxide in the mitochondrial matrix. Heterozygous SOD+/− mice exhibited mitochondrial oxidative damage and decreased mitochondrial membrane potential, along with significant elevation of 8-oxo-deoxyguanosine in both nuclear DNA and mtDNA [48]. Tumor incidence, in particular of lymphoma and pituitary adenoma, increased 100% in the old SOD2+/− mice compared with the wild-type mice (Table 1). These studies again suggest that defects in ROS-scavenging enzymes predispose cells to oncogenic transformation.45. Summary
Cancer cells exhibit an altered redox status and metabolism, which are associated with mitochondria as they are the major sites of ROS generation and energy metabolism. In this review, we have discussed mitochondrial dysfunction induced by the alterations of the mitochondrial genome, the TCA cycle enzymes, mitochondrial electron transport chain, and the associated oxidative stress. We have also discussed the aberrant oncogene and tumor suppressor signaling in the induction of mitochondrial dysfunction in cancers. Despite the prevalence of mtDNA mutations in different malignancies, their causal roles in cancer development remain incompletely understood and many questions still remain. It is imperative for us to gain a better understanding of why certain mutations appear more commonly in some cancers than in others. This may be in part due to the fact that different types of cancers have distinct underlying drivers and/or specific requirements in the path toward the malignant transformation. It has been known that oncogenic KRAS is the critical driver for the initiation and development of pancreatic cancers, while loss of tumor suppressors, such as PTEN and TP53, are major drivers of prostate cancer development. Mitochondrial dysfunction may be induced under either of these conditions but through distinct mechanisms. Certain control regions of mitochondrial single-nucleotide variants were recently found to co-occur with a gain of Myc oncogene in prostate cancer patients [11]. Different mtDNA mutations may impose divergent influences at varying degrees on the respiratory chain, with some mutations causing critical alterations of the respiratory chain and leading to transformation-promoting oxidative stress, while others may play lesser roles. The alterations of the electron transport chain affect not only electron transport, oxygen consumption, and ATP generation but also cellular redox status, metabolism, and apoptosis. It should be noted that a severe level of mitochondrial dysfunction may cause cell death and thus inhibit tumorigenesis, while mild mitochondrial dysfunction may enhance mitochondrial ROS generation and redox rebalance to stimulate cancer cell proliferation and invasiveness. Thus, a better understanding of the roles of mitochondrial dysfunction in cancer is essential for the future design of effective therapeutic strategies against diverse types of cancers.
Cancer cells exhibit an altered redox status and metabolism, which are associated with mitochondria as they are the major sites of ROS generation and energy metabolism. In this review, we have discussed mitochondrial dysfunction induced by the alterations of the mitochondrial genome, the TCA cycle enzymes, mitochondrial electron transport chain, and the associated oxidative stress. We have also discussed the aberrant oncogene and tumor suppressor signaling in the induction of mitochondrial dysfunction in cancers. Despite the prevalence of mtDNA mutations in different malignancies, their causal roles in cancer development remain incompletely understood and many questions still remain. It is imperative for us to gain a better understanding of why certain mutations appear more commonly in some cancers than in others. This may be in part due to the fact that different types of cancers have distinct underlying drivers and/or specific requirements in the path toward the malignant transformation. It has been known that oncogenic KRAS is the critical driver for the initiation and development of pancreatic cancers, while loss of tumor suppressors, such as PTEN and TP53, are major drivers of prostate cancer development. Mitochondrial dysfunction may be induced under either of these conditions but through distinct mechanisms. Certain control regions of mitochondrial single-nucleotide variants were recently found to co-occur with a gain of Myc oncogene in prostate cancer patients [86]. Different mtDNA mutations may impose divergent influences at varying degrees on the respiratory chain, with some mutations causing critical alterations of the respiratory chain and leading to transformation-promoting oxidative stress, while others may play lesser roles. The alterations of the electron transport chain affect not only electron transport, oxygen consumption, and ATP generation but also cellular redox status, metabolism, and apoptosis. It should be noted that a severe level of mitochondrial dysfunction may cause cell death and thus inhibit tumorigenesis, while mild mitochondrial dysfunction may enhance mitochondrial ROS generation and redox rebalance to stimulate cancer cell proliferation and invasiveness. Thus, a better understanding of the roles of mitochondrial dysfunction in cancer is essential for the future design of effective therapeutic strategies against diverse types of cancers.