Curcumin (CUR) is a hydrophobic polyphenolic compound found natively in turmeric. It exhibits antioxidant, antimicrobial, anti-inflammatory, pulmoprotective, anti-diabetic, hepatoprotective, nephroprotective, and antitumor actions. In addition to these pharmacological effects, CUR possesses neuroprotective activity where it protected the brain against oxidative injury induced by heavy metals.
- curcumin
- GSK-3β
- inflammation
- DNA damage
- oxidative stress
1. Introduction
Copper (Cu) is a redox-active metal found in many organs and tissues. It is essential for a plethora of biochemical processes such as blood clotting, iron absorption, protein homeostasis, energy production, and cellular metabolism [1]. It acts as a cofactor necessary for many redox-regulating proteins[2]. Cu homeostasis is maintained within the normal level by precise regulatory mechanisms that regulate its absorption, excretion, and blood level[3]. Genetic alteration in Cu-regulating ATPases, ATP7A, and ATP7B can cause Menkes disease (MD) and Wilson disease (WD), respectively [2][4][5]. MD is associated with a defect in Cu absorption and severe Cu deficiency, while WD results in Cu toxicity and affects several organs, including the liver, brain, and eye. Chronic exposure to Cu has been implicated in the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease [6]. Parkinson’s disease[7], and familial amyotrophic lateral sclerosis (ALS) [2][8].
Copper sulphate (CuSO4) is a well-known pesticide used for repelling pests that decreases the crop yield in agriculture. It is commonly used in tissue culture incubators to minimise the contamination risk as it has bactericidal and fungicidal properties. Accidental or intentional CuSO4 intoxication can induce multiorgan dysfunctions that could be fatal. The systemic absorption of Cu occurs through the gastrointestinal tract, lungs, and skin[9]. The clinical manifestations of Cu toxicity are erosive gastropathy, acute liver and kidney injuries, intravascular hemolysis, arrhythmia, rhabdomyolysis, and seizures [10]. Although the mechanisms of CuSO4 toxicity are not fully addressed, they represent a combination of significant oxidative stress and endocrine perturbation in the vulnerable organs of the body[11]. Animal studies showed that the chronic oral administration of CuSO4 causes liver and kidney functional impairment due to increased Cu levels in the respective organs [12]. The toxic effects of Cu on the liver and kidney have been studied extensively, while the toxicities of other vital organs of the body are less documented. Similar to other metals, the management of Cu toxicity includes the use of chelating agents such as D-penicillamine, tetrathiomolybdate, and trientine[13]. Other chelators such as deferoxamine (DFO) have an affinity for Cu binding[14]. Despite the effectiveness of these chelators, they often associated with some serious adverse effects on cardiovascular, gastrointestinal, respiratory, and nervous systems, which necessitates the use of safer alternatives. In addition, the limited or moderate effectiveness of these chelators has been found in some cases.
2. Results
2. N-CUR and CUR Attenuate Cu-Induced Cerebral Oxidative Stress
2.1. N-CUR and CUR Attenuate Cu-Induced Cerebral Oxidative Stress
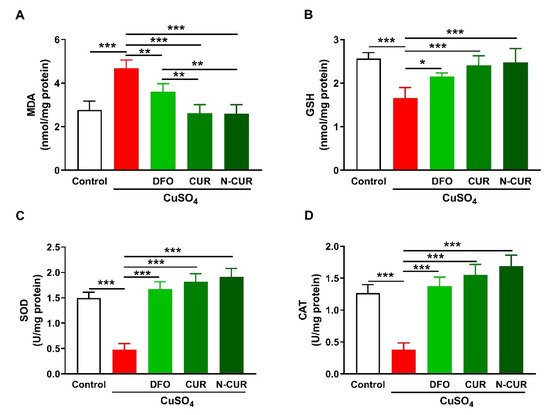
3. N-CUR and CUR Suppress Cerebral Inflammation in Cu-Administered Rats
2.2. N-CUR and CUR Suppress Cerebral Inflammation in Cu-Administered Rats
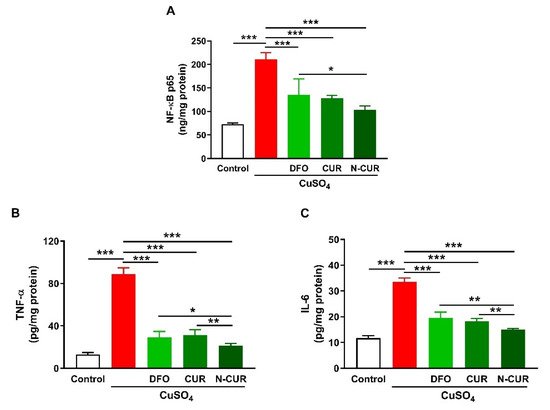
4. N-CUR and CUR Prevent Apoptosis in Cu-Administered Rats
2.3. N-CUR and CUR Prevent Apoptosis in Cu-Administered Rats
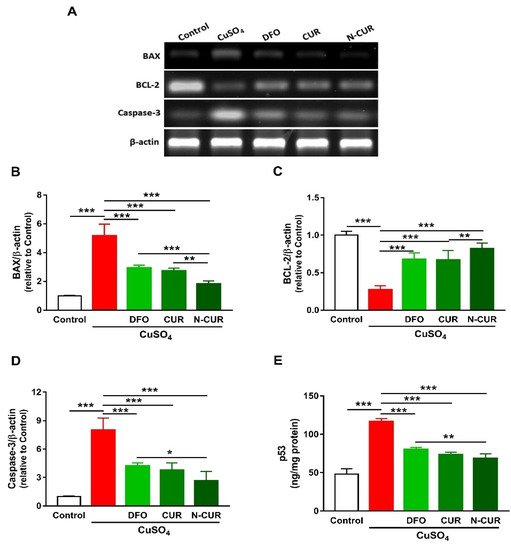
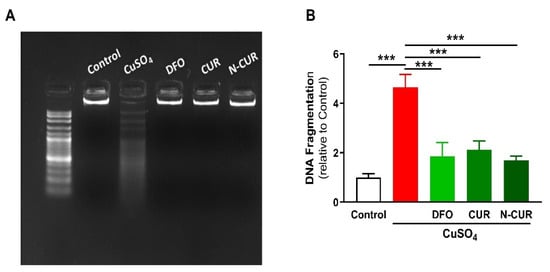
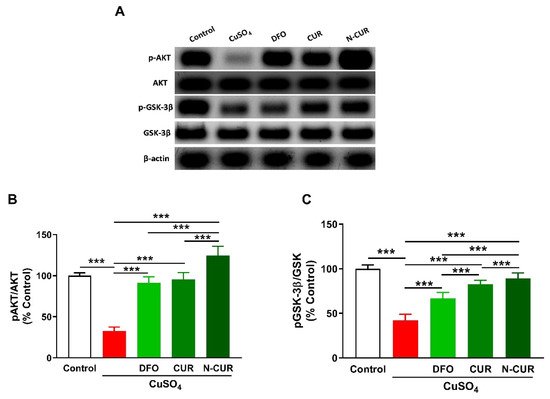
5. N-CUR and CUR Upregulate AKT/GSK-3β Signaling in Cu-Administered Rats
2.4. N-CUR and CUR Upregulate AKT/GSK-3β Signaling in Cu-Administered Rats
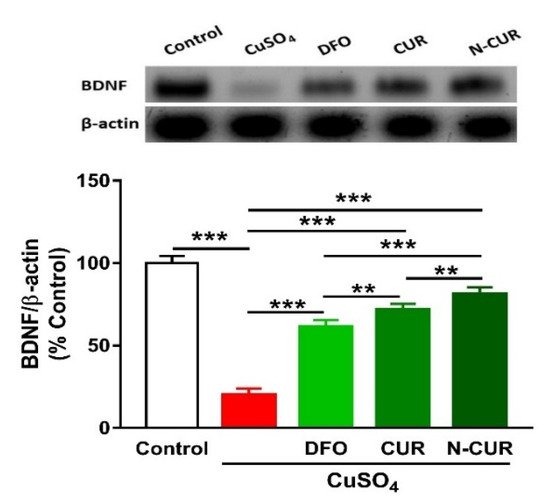
6. N-CUR and CUR Upregulate Brain-Derived Neurotrophic Factor (BDNF) in Cu-Administered Rats
2.5. N-CUR and CUR Upregulate Brain-Derived Neurotrophic Factor (BDNF) in Cu-Administered Rats
73. Conclusions
References
- Scheiber, I.F.; Mercer, J.F.; Dringen, R. Metabolism and functions of copper in brain. Prog. Neurobiol. 2014, 116, 33–57. [Google Scholar] [CrossRef]
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper that cancer’. Met. Integr. Biometal Sci. 2015, 7, 1459–1476. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-E.; Nevitt, T.; Thiele, D.J. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper signalling: Causes and consequences. Cell Commun. Signal 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J. Alzheimer’s disease causation by copper toxicity and treatment with zinc. Front Aging Neurosci. 2014, 6, 92. [Google Scholar] [CrossRef]
- Montes, S.; Rivera-Mancia, S.; Diaz-Ruiz, A.; Tristan-Lopez, L.; Rios, C. Copper and copper proteins in parkinson’s disease. Oxidative Med. Cell. Longev. 2014, 2014, 147251. [Google Scholar] [CrossRef]
- Bourassa, M.W.; Brown, H.H.; Borchelt, D.R.; Vogt, S.; Miller, L.M. Metal-deficient aggregates and diminished copper found in cells expressing sod1 mutations that cause als. Front Aging Neurosci. 2014, 6, 110. [Google Scholar] [CrossRef]
- Hashish, E.A.; Elgaml, S.A. Hepatoprotective and nephroprotective effect of curcumin against copper toxicity in rats. Indian J. Clin. Biochem. IJCB 2016, 31, 270–277. [Google Scholar] [CrossRef]
- Gamakaranage, C.S.; Rodrigo, C.; Weerasinghe, S.; Gnanathasan, A.; Puvanaraj, V.; Fernando, H. Complications and management of acute copper sulphate poisoning; a case discussion. J. Occup. Med. Toxicol. 2011, 6, 34. [Google Scholar] [CrossRef]
- Rana, S.V. Perspectives in endocrine toxicity of heavy metals--a review. Biol. Trace Elem. Res. 2014, 160, 1–14. [Google Scholar] [CrossRef]
- Kumar, V.; Kalita, J.; Misra, U.K.; Bora, H.K. A study of dose response and organ susceptibility of copper toxicity in a rat model. J. Trace Elem. Med. Biol. 2015, 29, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Tegoni, M.; Valensin, D.; Toso, L.; Remelli, M. Copper chelators: Chemical properties and bio-medical applications. Curr. Med. Chem. 2014, 21, 3785–3818. [Google Scholar] [CrossRef]
- Lawson, M.K.; Valko, M.; Cronin, M.T.D.; Jomová, K. Chelators in iron and copper toxicity. Curr. Pharm. Rep. 2016, 2, 271–280. [Google Scholar] [CrossRef]
