Depression and anxiety are the most common psychiatric disorders in end-stage renal disease (ESRD) patients treated with hemodialysis (HD) and may correlate with lower quality of life and increased mortality. Depression treatment in HD patients is still a challenge both for nephrologists and psychiatrists. The possible treatment of depressive disorders can be pharmacological and non-pharmacological. In our article, we focus on the use of sertraline, the medication which seems to be relatively safe and efficient in the abovementioned population, taking under consideration several limitations regarding the use of other selective serotonin reuptake inhibitors (SSRIs).
- depression
- hemodialysis
- sertraline
Note: The entry will be online only after author check and submit it.
1. Introduction
Depression and anxiety are the most common psychiatric disorders in end-stage renal disease (ESRD) patients treated with hemodialysis (HD). Their prevalence is increasing in recent years [1], reaching the rate of 20–40% for depression [2,3] and 20–52% for anxiety [4], depending on the methodological approach. According to the studies, these psychiatric disorders may correlate with lower quality of life, increased hospitalization rate, suicidal behavior, hemodialysis nonadherence, and increased mortality [5]. Despite the number of studies on this issue, major depressive disorder (MDD) in ESRD patients is still underdiagnosed, and its treatment is not optimal [6] [Table 1]. Anxiety disorders seem to be overlooked even more often [7], which is probably associated with significant overlapping of symptoms with depression [8]. As initiation of dialysis is a large change in everyday functioning with a potential to induce or worsen psychiatric symptoms, screening for depression [9] and anxiety is suggested at the beginning of renal replacement treatment.
Depression and anxiety are the most common psychiatric disorders in end-stage renal disease (ESRD) patients treated with hemodialysis (HD). Their prevalence is increasing in recent years [1], reaching the rate of 20–40% for depression [2][3] and 20–52% for anxiety [4], depending on the methodological approach. According to the studies, these psychiatric disorders may correlate with lower quality of life, increased hospitalization rate, suicidal behavior, hemodialysis nonadherence, and increased mortality [5]. Despite the number of studies on this issue, major depressive disorder (MDD) in ESRD patients is still underdiagnosed, and its treatment is not optimal [6] [Table 1]. Anxiety disorders seem to be overlooked even more often [7], which is probably associated with significant overlapping of symptoms with depression [8]. As initiation of dialysis is a large change in everyday functioning with a potential to induce or worsen psychiatric symptoms, screening for depression [9] and anxiety is suggested at the beginning of renal replacement treatment.
| SSRI | SNRI | SARI | SNRISA | SMS | TCA |
|---|---|---|---|---|---|
| [ | 11 | ] | |||
| Citalopram | Desvenlafaxine | Nefazodone | Amoxapine | ||
| Main symptoms |
|
|
|||
| Protriptyline | |||||
| Trimipramine |
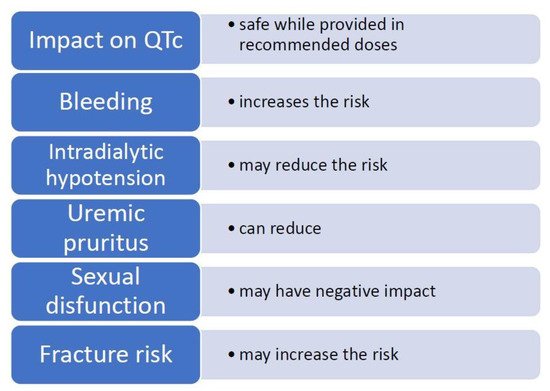
| Impact on QTc Prolongation | Safe While Provided in Recommended Doses [20] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vilazodone | |||||||||||
| Bleeding | Increases the risk of bleeding [21 | Amitriptyline | |||||||||
| , | 22][21][22] | Escitalopram | Duloxetine | ||||||||
| Platelet reactivity | May reduce platelet activation [23,24,25] | Trazodone | [ | Vortioxetine | Clomipramine | ||||||
| 23 | ][24][25] | Additional symptoms |
|
Fluoxetine | Levomilnacipram | Desipramine | |||||
| Intradialytic hypotension (IDH) | Inconsistent study results, may reduce the risk of IDH [26,27,28,29,30][26][27][28][29][30] |
|
|||||||||
| Diagnostic criteria | At least two main symptoms and additional symptoms in a total number of at least four [18 | Fluvoxamine | ][10] | At least one main symptom and additional symptoms in a total number of at least | |||||||
| Milnacipran | 31,32,33] | Doxepin | Duration of symptoms | At least two weeks [18][10] | At least two weeks | ||||||
| Chronic kidney disease-associated pruritus (CKD-aP) | Can reduce pruritus in cases caused by CKD [[31][32][33] | Paroxetine | |||||||||
| Cytokines | Reduces the concentration of pro-inflammatory and increases the levels of anti-inflammatory cytokines, insufficient data in HD population [34,35,36][ | [19] | Venlafaxine | [11] | |||||||
| 34][35][36 | Imipramine | ] | Severity of symptoms | Clinical differentiation:
| |||||||
| Sertraline |
| ||||||||||
| Sexual disfunction (SD) | Negative impact on SD in general population [37,38][37][38] and in HD population [39The symptoms ought to cause significant impairment in social, occupational or another important area of functioning | [19][11] | |||||||||
| Nortriptyline | ] | Exclusion criteria |
|
||||||||
| Fracture risk and osteoporosis | Is associated with hip fracture risk and may decrease bone mass in general population [40,41][40 |
| |||||||||
| ][41], may increase fracture risk in ESRD population [42] |
2. Sertraline—General Properties of the Medication
References
- Saran, R.; Robinson, B.; Abbott, K.C.; Bragg-Gresham, J.; Chen, X.; Gipson, D.; Gu, H.; Hirth, R.A.; Hutton, D.; Jin, Y.; et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2020, 75 (Suppl. S1), A6–A7.
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013, 84, 179.
- King-Wing Ma, T.; Kam-Tao Li, P. Depression in dialysis patients. Nephrology 2016, 21, 639–646.
- Murtagh, F.E.; Addington-Hall, J.; Higginson, I.J. The prevalence of symptoms in end-stage renal disease: A systematic review. Adv. Chronic Kidney Dis. 2007, 14, 82–99.
- Bautovich, A.; Katz, I.; Smith, M.; Loo, C.K.; Harvey, S.B. Depression and chronic kidney disease: A review for clinicians. Aust. N. Z. J. Psychiatry 2014, 48, 530–541.
- Hedayati, S.S.; Yalamanchili, V.; Finkelstein, F.O. A practical approach to the treatment of depression in patients with chronic kidney disease and end-stage renal disease. Kidney Int. 2012, 81, 247–255.
- Schouten, R.W.; Haverkamp, G.L.; Loosman, W.L.; Chandie Shaw, P.K.; van Ittersum, F.J.; Smets, Y.F.C.; Vleming, L.J.; Dekker, F.W.; Honig, A.; Siegert, C.E.H. Anxiety symptoms, mortality and hospitalization in patients receiving maintenance dialysis: A cohort study. Am. J. Kidney Dis. 2019, 74, 158–166.
- Cohen, S.D.; Cukor, D.; Kimmel, P.L. Anxiety in Patients Treated with Hemodialysis. Clin. J. Am. Soc. Nephrol. 2016, 11, 2250–2255.
- Cohen, S.D.; Norris, L.; Aquaviva, K.; Peterson, R.A.; Kimmel, P.L. Screening, diagnosis and treatment of depression in patients with end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2007, 2, 133–142.
- World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research; World Health Organization: Geneva, Switzerland, 1993; p. 248.
- American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Arlington, VA, USA, 2013. Available online: https://www.ncjrs.gov/App/Publications/abstract.aspx?ID=271642 (accessed on 1 September 2021).
- Fasipe, O.J. Neuropharmacological classification of antidepressant agents based on their mechanisms of action. Arch. Med. Health Sci. 2018, 6, 81–94.
- Hughes, Z.A.; Starr, K.R.; Langmead, C.J.; Hill, M.; Bartoszyk, G.D.; Hagan, J.J.; Middlemiss, D.N.; Dawson, L.A. Neurochemical evaluation of the novel 5-HT1A receptor partial agonist/serotonin reuptake inhibitor, vilazodone. Eur. J. Pharmacol. 2005, 510, 49–57.
- Constantino, J.L.; Fonseca, V.A. Pharmacokinetics of antidepressants in patients undergoing hemodialysis: A narrative literature review. Braz. J. Psychiatry 2019, 41, 441–446.
- Jaber, B.L.; Lee, Y.; Collins, A.J.; Hull, A.R.; Kraus, M.A.; McCarthy, J.; Miller, B.W.; Spry, L.; Finkelstein, F.O. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: Interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. 2010, 56, 531–539.
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: A systematic review and network meta-analysis. Lancet 2018, 391, 1357–1366.
- US Food and Drug Administration. FDA Drug Safety Communication: Revised Recommendations for Celexa (Citalopram Hydrobromide) Related to a Potential Risk of Abnormal Heart Rhythms with High Doses. US Food and Drug Administration (2012). Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-revised-recommendations-celexa-citalopram-hydrobromide-related (accessed on 1 September 2021).
- Health Canada 2012 Health CanadaAntidepressant cipralex (Escitalopram): Updated information Regarding Dose-Related Heart Risk. 2012. Available online: http://healthycanadians.gc.ca/recall-alert-rappel-avis/hc-sc/2012/13674a-eng.php (accessed on 3 January 2021).
- Crépeau-Gendron, G.; Brown, H.K.; Shorey, C.; Madan, R.; Szabuniewicz, C.; Koh, S.; Veinish, S.; Mah, L. Association between citalopram, escitalopram and QTc prolongation in a real-world geriatric setting. J. Affect. Disord. 2019, 250, 341–345.
- Assimon, M.M.; Brookhart, M.A.; Flythe, J.E. Comparative Cardiac Safety of Selective Serotonin Reuptake Inhibitors among Individuals receiving Maintenance Hemodialysis. J. Am. Soc. Nephol. 2019, 30, 611–623.
- Jiang, H.Y.; Chen, H.Z.; Hu, X.J.; Yu, Z.H.; Yang, W.; Deng, M.; Zhang, Y.H.; Ruan, B. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2015, 13, 42–50.
- Iwagami, M.; Tomlinson, L.A.; Mansfield, K.E.; Douglas, I.J.; Smeeth, L.; Nitsch, D. Gastrointestinal bleeding risk of selective serotonin reuptake inhibitors by level of kidney function: A population-based cohort study. Br. J. Clin. Pharmacol. 2018, 84, 2142–2151.
- Lopez-Vilchez, I.; Serra-Millas, M.; Navarro, V.; Rosa Hernandez, M.; Villalta, J.; Diaz-Ricart, M.; Gasto, C.; Escolar, G.; Galan, A.M. Prothrombotic platelet phenotype in major depression: Downregulation by antidepressant treatment. J. Affect. Disord. 2014, 159, 39–45.
- Tseng, Y.L.; Chiang, M.L.; Lane, H.Y.; Su, K.P.; Lai, Y.C. Selective serotonin reuptake inhibitors reduce P2Y12 receptor-mediated amplification of platelet aggregation. Thromb. Res. 2013, 131, 325–332.
- Acedillo, R.R.; Shah, M.; Devereaux, P.J.; Li, L.; Iansavichus, A.V.; Walsh, M.; Garg, A.X. The risk of perioperative bleeding in patients with chronic kidney disease: A systematic review and meta-analysis. Ann. Surg. 2013, 258, 901–913.
- Molin, C.Z.Z.D.; Sakae, T.M.; Schuelter-Trevisol, F.; Trevisol, D.J. Effects of sertraline in the prevention of low blood pressure in patients undergoing hemodialysis. J. Bras. Nefrol. 2019, 41, 492–500.
- Razeghi, E.; Dashti-Khavidaki, S.; Nassiri, S.; Abolghassemi, R.; Khalili, H.; Nazari, S.S.H.; Mansournia, A.M.; Taraz3, M. A randomized crossover clinical trial of sertraline for intradialytic hypotension. Iran. J. Kidney Dis. 2015, 9, 323–330.
- Yalcin, A.U.; Sahin, G.; Erol, M.; Bal, C. Sertraline hydrochloride treatment for patients with hemodialysis hypotension. Blood Purif. 2002, 20, 150–153.
- Yalcin, A.U.; Kudaiberdieva, G.; Sahin, G.; Gorenek, B.; Akcar, N.; Kuskus, S.; Bayrak, F.; Timuralp, B. Effect of sertraline hydrochloride on cardiac autonomic dysfunction in patients with hemodialysis-induced hypotension. Nephron Physiol. 2003, 93, 21–28.
- Brewster, U.C.; Ciampi, M.A.; Abu-Alfa, A.K.; Perazella, M.A. Addition of sertraline to other therapies to reduce dialysis-associated hypotension. Nephrology 2003, 8, 296–301.
- Shakiba, M.; Sanadgol, H.; Azmoude, H.R.; Mashhadi, M.A.; Sharifi, H. Effect of sertraline on uremic pruritus improvement in ESRD patients. Int. J. Nephrol. 2012, 2012, 363901.
- Chan, K.Y.; Li, C.W.; Wong, H.; Yip, T.; Chan, M.L.; Cheng, H.W.; Sham, M.K. Use of sertraline for antihistamine-refractory uremic pruritus in renal palliative care patients. J. Palliat. Med. 2013, 16, 966–970.
- Pakfetrat, M.; Malekmakan, L.; Hashemi, N.; Tadayon, T. Sertraline can reduce uremic pruritus in hemodialysis patient: A double blind randomized clinical trial from Southern Iran. Hemodial. Int. 2018, 22, 103–109.
- Al-Hakeim, H.K.; Twayej, A.J.; Al-Dujaili, A.H. Reduction in serum IL-1β IL-6, and IL-18 levels and Beck Depression Inventory-II score by combined sertraline and ketoprofen administration in major depressive disorder: A clinical trial. Neurol. Psychiatry Brain Res. 2018, 30, 148–153.
- Sutcigil, L.; Oktenli, C.; Musabak, U.; Bozkurt, A.; Cansever, A.; Uzun, O.; Sanisoglu, S.Y.; Yesilova, Z.; Ozmenler, N.; Ozsahin, A.; et al. Pro- and anti-inflammatory cytokine balance in major depression: Effect of sertraline therapy. Clin. Dev. Immunol. 2007, 2007, 1–6.
- Taraz, M.; Khatami, M.R.; Dashti-Khavidaki, S.; Akhonzadeh, S.; Noorbala, A.A.; Ghaeli, P.; Taraz, S. Sertraline decreases serum level of interleukin-6 (IL-6) in hemodialysis patients with depression: Results of a randomized double-blind, placebo-controlled clinical trial. Int. Immunopharmacol. 2013, 17, 917–923.
- Khazaie, H.; Rezaie, L.; Rezaei Payam, N.; Najafi, F. Antidepressant-induced sexual dysfunction during treatment with fluoxetine, sertraline and trazodone; a randomized controlled trial. Gen. Hosp. Psychiatry 2015, 37, 40–45.
- Serretti, A.; Chiesa, A. Treatment-emergent sexual dysfunction related to antidepressants: A meta-analysis. J. Clin. Psychopharmacol. 2009, 29, 259–266.
- Palmer, S.C.; Natale, P.; Ruospo, M.; Saglimbene, V.M.; Rabindranath, K.S.; Craig, J.C.; Strippoli, G.F. Antidepressants for treating depression in adults with end-stage kidney disease treated with dialysis. Cochrane Database Syst. Rev. 2016, 5, CD004541.
- Khanassov, V.; Hu, J.; Reeves, D.; van Marwijk, H. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: A systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 2018, 33, 1688–1708.
- Rabenda, V.; Nicolet, D.; Beaudart, C.; Bruyère, O.; Reginster, J.-Y. Relationship between use of antidepressants and risk of fractures: A meta-analysis. Osteoporos. Int. 2013, 24, 121–137.
- Vangala, C.; Niu, J.; Montez-Rath, M.E.; Yan, J.; Navaneethan, S.D.; Winkelmayer, W.C. Selective Serotonin Reuptake Inhibitor Use and Hip Fracture Risk Among Patients on Hemodialysis. Am. J. Kidney Dis. 2020, 75, 351–360.
- Nagler, E.V.; Webster, A.C.; Vanholder, R.; Zoccali, C. Antidepressants for depression in stage 3–5 chronic kidney disease: A systematic review of pharmacokinetics, efficacy and safety with recommendations by European Renal Best Practice (ERBP). Nephrol. Dial. Transplant. 2012, 27, 3736–3745.
- Hirschfeld, R.M.; Montgomery, S.A.; Aguglia, E.; Amore, M.; Delgado, P.L.; Gastpar, M.; Hawley, C.; Kasper, S.; Linden, M.; Massana, J.; et al. Partial response and nonresponse to antidepressant therapy: Current approaches and treatment options. J. Clin. Psychiatry. 2002, 63, 826–837.
- Fredman, S.J.; Fava, M.; Kienke, A.S.; White, C.N.; Nierenberg, A.A.; Rosenbaum, J.F. Partial response, nonresponse, and relapse with selective serotonin reuptake inhibitors in major depression: A survey of current “next-step” practices. J. Clin. Psychiatry 2000, 61, 403–408.
- Labbate, L.A.; Fva, M.; Rosenbaum, J.F.; Avana, G.W. Drugs for the treatment of depression. In Handbook of Psychiatric Drug Therapy, 6th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010; p. 54.
- Schwenk, M.H.; Verga, M.A.; Wagner, J.D. Hemodialyzability of sertraline. Clin. Nephrol. 1995, 44, 121–124.
- Brunton, L.; Chabner, B.; Knollman, B. Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 12th ed.; McGraw Hill Professional: New York, NY, USA, 2010; ISBN 9780071769396.
- DeVane, C.L.; Liston, H.L.; Markowitz, J.S. Clinical pharmacokinetics of sertraline. Clin. Pharmacokinet. 2002, 41, 1247–1266.
- Hu, X.H.; Bull, S.A.; Hunkeler, E.M.; Ming, E.; Lee, J.Y.; Fireman, B.; Markson, L.E. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: Patient report versus physician estimate. J. Clin. Psychiatry 2004, 65, 959.
- Serebruany, V.L.; Glassman, A.H.; Malinin, A.I.; Nemeroff, C.B.; Musselman, D.L.; van Zyl, L.T.; Finkel, M.S.; Krishnan, K.R.; Gaffney, M.; Harrison, W.; et al. Sertraline AntiDepressant Heart Attack Randomized Trial Study Group. Platelet/endothelial biomarkers in depressed patients treated with the selective serotonin reuptake inhibitor sertraline after acute coronary events: The Sertraline AntiDepressant Heart Attack Randomized Trial (SADHART) Platelet Substudy. Circulation 2003, 108, 939–944.
- Labos, C.; Dasgupta, K.; Nedjar, H.; Turecki, G.; Rahme, E. Risk of bleeding associated with combined use of selective serotonin reuptake inhibitors and antiplatelet therapy following acute myocardial infarction. CMAJ 2011, 183, 1835–1843.
- Halperin, D.; Reber, G. Influence of antidepressants on hemostasis. Dialogues Clin. Neurosci. 2007, 9, 47–59.
- Sienaert, P. Managing the Adverse Effects of Antidepressants. Psychiatr. Times 2014, 31. Available online: https://www.psychiatrictimes.com/view/managing-adverse-effects-antidepressants (accessed on 25 January 2021).
- Stryjer, R.; Spivak, B.; Strous, R.D.; Shiloh, R.; Harary, E.; Polak, L.; Birgen, M.; Kotler, M.; Weizman, A. Trazodone for the treatment of sexual dysfunction induced by serotonin reuptake inhibitors: A preliminary open-label study. Clin. Neuropharmacol. 2009, 32, 82–84.
- Keller, M.B. The long-term treatment of depression. J. Clin. Psychiatry 1999, 60 (Suppl. S17), 41–45.
