One-third of patients with cirrhosis present kidney failure (AKI and CKD). It has multifactorial causes and a harmful effect on morbidity and mortality before and after liver transplantation. Kidney function does not improve in all patients after liver transplantation, and liver transplant recipients are at a high risk of developing chronic kidney disease. The causes of renal dysfunction can be divided into three groups: pre-operative, perioperative and post-operative factors.
1. Kidney Function and Cirrhosis
Cirrhosis is now the 4th leading cause of death in Europe and the main indication for liver transplantation
[1]. Regardless of the initial disease, the prognosis for patients with cirrhosis is saddled with numerous complications, one of which is kidney failure. Kidney failure can occur as acute kidney injury with or without dialysis requirement, chronic kidney disease or end-stage kidney disease. It occurs in one-third of patients with cirrhosis
[2][3][2,3]. Chronic kidney disease (CKD) is responsible for increased mortality
[4]. The mechanisms responsible for renal dysfunction in patients with cirrhosis are multifactorial and include a combination of functional and organic renal impairments
[5], such as vasodilation of the splanchnic arterial system and intestinal bacterial translocation secondary to the portal hypertension (PHT). These phenomena are responsible for hepatorenal syndrome (HRS)
[6]. In addition, there are renal damages associated with cardiovascular morbidities (diabetes, insulin resistance, hypertension and obesity), renal damage specific to cirrhosis or the initial liver disease (IgA nephropathy, cryoglobulinemic membranoproliferative glomerulonephritis (MPGN)) or repeated renal toxicity of drugs and contrast agents
[7]. All these factors contribute to the occurrence of irreversible lesions and impact renal function. It is now widely accepted that pre-transplantation kidney function is a major prognostic factor in pre-transplant mortality
[3].
2. Change in Kidney Function Post-Transplantation
The change in kidney function after transplantation is extremely variable and depends on many factors. In general, kidney function deteriorates rapidly during the first year, stabilizes and then decreases more gradually
[8][10].
2.1. Acute Kidney Injury after Liver Transplantation
The risk of post-transplant AKI is significant. However, the variability in incidence across studies is due to the lack of consensus on the diagnostic criteria. In 2004, the second ADQI (Acute Dialysis Quality Initiative) group consensus conference established the RIFLE (Risk of kidney dysfunction, Injury to the kidney, failure of kidney function, loss of kidney function, and end-stage renal disease) classification based on changes in GFR and diuresis, adopted in 2010 by the ICA (International Club of Ascites) for patients with cirrhosis, regardless of the cause of kidney failure. In 2011, the AKIN (Acute Kidney Injury Network) group improved the RIFLE criteria to produce the AKIN classification (
Appendix A). With these more sensitive criteria, it takes into account less severe renal impairments and, therefore, increases the incidence of AKI
[9][8]. The ADQI-ICA committee suggests the use of AKIN criteria in patients with cirrhosis for the diagnosis of AKI
[10][9].
A review of 67 observational studies published between 1985 and 2019 showed that over 50% of transplant patients will develop AKI within the early period after transplantation
[9][10][11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][8,9,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Fifteen percent will require dialysis
[51][70][51,70]. Ninety-five percent (95%) of the cases of AKI develop within 7 days of transplantation and are usually attributed to a high MELD score, the presence of ascites or prolonged perioperative hypotension
[36]. Late post-operative AKI that occurs after the first week is mainly associated with infectious complications, repeat surgery, or delayed or no recovery of liver function. The recovery rate after AKI with or without the need for dialysis is between 60% and 80%
[24][25][24,25]. This seems to be associated with the existence of a pre-transplantation hepato-renal syndrome and can occur from a few days to several months after surgery. However, AKI causes a significant risk of CKD which is even greater with a longer period of hemodialysis.
2.2. Post-Transplantation Chronic Kidney Disease and End-Stage Renal Disease
CKD is common after organ transplantation, mainly during recovery from liver transplantation
[70]. A review of 46 observational studies between 1990 and 2019
[15][20][24][28][31][43][49][50][51][66][71][72][73][74][75][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][15,20,24,28,31,43,49,50,51,66,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105] showed that CKD is observed in 10% to 75% of liver-transplant patients beyond 20 years post-transplantation. This wide variability is due to inhomogeneous definitions, measurement methods, and lengths of follow up between studies. Some use creatinine levels, while others use GFR thresholds or variations. At one year post-transplantation, an average of 19% of patients develops CKD compared to 29% and 69%, respectively, at 5 and 15 years post-transplantation. The incidence of end-stage renal disease follows the same course (an average of 4% and 11%, respectively, at 5 and 15 years post-transplantation). This decrease in GFR has an exponential impact on post-transplantation mortality
[93] with an overall risk of mortality increased by a factor of four in the event of CKD
[106]. The mechanisms responsible for post-transplantation impairment of kidney function are multifactorial with variable histological manifestations.
2.3. The Contribution of Histology in Post-Transplantation Kidney Failure
Few studies have examined histological renal lesions in liver transplant patients with kidney failure. However, these analyses confirm that renal lesions are numerous, varied, complex and multifactorial. In contrast to renal histological lesions in cirrhotic patients, few specific primary glomerular lesions are detected. Most of the damage consists of vascular lesions, tubular necrosis or non-specific glomerular lesions (thickening of the glomerular basement membrane, fibrous expansion of the mesangium, segmental and focal hyalinosis lesions, nodular diabetic glomerulosclerosis or podocyte depletion observed under electron microscopy)
[107]. In 15% to 50% of the cases, there are lesions secondary to calcineurin inhibitor toxicity characterized histologically by nodular arteriolar hyalinosis and/or interstitial fibrosis in bands
[108]. Rare cases of membranous glomerulonephritis, oxalate nephropathy or BK virus nephropathy have also been described
[109][110][109,110]. The high variability of post-transplantation lesions observed and the low rate of pre-transplantation histological lesions suggest the occurrence of new nephrotoxic events
[107]. Given the high number of non-specific lesions, histological evaluation constitutes a challenge in the post-transplantation quantification of these lesions. In previous studies, we suggested evaluating the percentage of glomerular obsolescence; acute tubular necrosis; and according to the Banff classification, interstitial fibrosis (ci), tubular atrophy (ct) and intimal thickening (cv)
[80][111][80,111]. Similarly, Kubal et al. propose a prognostic histological score based on the semi-quantitative staging of histological lesions, such as inflammation, tubular atrophy, interstitial fibrosis, intimal thickness and mesangial thickening. They showed that this histological score is highly correlated with renal survival. It is interesting to note that severe histological lesions, such as glomerulosclerosis, fibrous endarteritis and interstitial fibrosis, were even observed in patients with normal kidney function (GFR ≥ 60 mL/min/1.73 m
2)
[108].
2.4. The Impact of Kidney Function on Post-Transplantation Mortality
Most studies report a strong relationship between kidney failure and patient survival
[93][112][93,112]. In fact, as is the case in pre-transplantation, post-transplant renal dysfunction is an independent predictor of immediate and long-term mortality in liver transplant patients
[44][47][94][113][44,47,94,113]. Mainly in AKI that occurs immediately after transplantation, the need for post-operative dialysis is associated with a higher risk of mortality
[18][55][18,55]. Similarly, persistent CKD after liver-transplantation increases the short-term risk of mortality (≤1 year) by a factor of three (2.55 to 3.20 depending on the study)
[36][114][36,114], as well as the long-term risk of mortality, as highlighted by a meta-analysis by Fabrizi et al., who showed an increased mortality by a factor of four
[106]. This increases with the severity of renal dysfunction, if the onset is late (>1 year after transplantation) or if the patient requires dialysis
[113].
Recently, Allen et al. established a mortality risk assessment model based on measured or estimated GFR and creatinine values in a cohort of 1211 liver transplant recipients monitored for 25 years
[72]. When GFR decreases below 60 mL/min/1.73 m
2, mortality increases exponentially (the relative risk of death increases by up to 5.5 times). Using serum creatinine, they found a U-shaped curve with an excess risk of mortality for extreme values (<0.75 and >2 mg/dL).
3. Factors Associated with the Risk of Post-Transplantation Renal Dysfunction
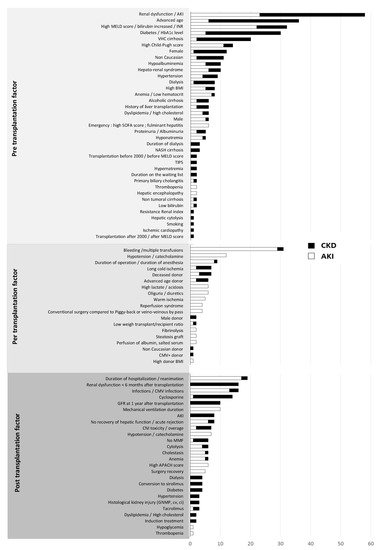
The cause of renal dysfunction after liver transplantation is multifactorial. There are many risk factors involved in AKI and CKD
[66][78][111][66,78,111]. However, they can be classified into three categories: pre-transplantation, perioperative and post-operative. We identified these predictive factors by uni- or multivariate analysis from 101 observational studies published between 1996 and 2019
[9][8][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][36][37][38][40][41][42][43][44][45][46][48][53][56][60][61][62][63][64][70][71][73][76][77][78][79][80][81][82][83][84][85][86][87][89][90][91][92][93][94][95][96][97][104][106][107][108][111][112][113][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][8,10,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,36,37,38,40,41,42,43,44,45,46,48,53,56,60,61,62,63,64,70,71,73,76,77,78,79,80,81,82,83,84,85,86,87,89,90,91,92,93,94,95,96,97,104,106,107,108,111,112,113,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136] (
Figure 1).
Figure 1. Inventory of the pre-transplantation, perioperative and post-operative risk factors for AKI or CKD according to the number of studies that mention them
[9][8][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][36][37][38][40][41][42][43][44][45][46][48][53][56][60][61][62][63][64][70][71][73][76][77][78][79][80][81][82][83][84][85][86][87][89][90][91][92][93][94][95][96][97][104][106][107][108][111][112][113][115][116][117][118][119][120][121][122][123][124][125][126][127][128][129][130][131][132][133][134][135][136][8,10,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,36,37,38,40,41,42,43,44,45,46,48,53,56,60,61,62,63,64,70,71,73,76,77,78,79,80,81,82,83,84,85,86,87,89,90,91,92,93,94,95,96,97,104,106,107,108,111,112,113,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136]. AKI: acute kidney injury; CKD: chronic kidney disease.
4. Markers and Predictive Models of Post-Transplantation Renal Dysfunction
Therefore, there are many risk factors that expose patients to post-transplantation CKD. Some factors are non-modifiable (age, hepatopathy or cardiovascular comorbidity), and it is, therefore, crucial to determine modifiable and preventable risk factors. Consequently, over the past 10 years, new concepts have focused on the detection of non-invasive markers of nephrotoxicity and the establishment of predictive models of post-transplantation CKD. This enables the pre-transplantation or early post-transplantation identification of patients at high risk for renal dysfunction in order to prevent kidney failure as much as possible
[137][144].
4.1. Markers of Post-Transplantation Renal Dysfunction
Early post-transplantation serum creatinine (D5) is associated with an excess risk of CKD
[66]. The use of biomarkers (blood or urine) is an interesting innovative approach for early diagnosis, characterization of renal impairment (pre or intrarenal) and quantification of irreversible renal lesions
[138][145]. Among the most promising are interleukin 18 (IL-18), kidney injury molecule 1 (KIM-1), liver-type fatty acid binding protein (L-FABP), osteopontin, TIMP-2 and neutrophil gelatinase-associated lipocalin (NGAL). In patients with cirrhosis, an elevation in these biomarkers seems to be more closely associated with structural kidney lesions than with functional impairment
[139][146]. In certain studies, they are even considered as predictive factors of AKI after liver transplantation, but the results remain inconsistent. However, some plasma biomarkers, such as NGAL, are associated with a risk of early post-transplantation CKD
[117]. Similarly, Levitsky et al. showed an association between a specific plasma proteome profile and the development of chronic kidney disease after transplantation
[126]. However, these promising tools are not yet validated and at present remain in the realm of research
[140][147].
4.2. Long-Term CKD Prediction Models
In order to improve the identification of patients at risk, certain teams have developed formulas to predict the risk of CKD after liver transplantation. Giusto et al. established and validated a model for predicting CKD 12 months post-transplantation, based on a regression analysis that takes into account GFR, hypertension and episodes of severe infection
[78]. However, this model is limited by a small number of variables that contrast with all the risk factors identified to date. Israni et al. propose a model for ESRD prediction at 6 months and 5 years post-transplantation. This model takes into account more variables, such as age, history of diabetes, cancer or dialysis, ethnicity, hepatitis C status, BMI, donor risk index, albumin, bilirubin and creatinine levels. It has the advantage of being more complete and, therefore, more precise in terms of risk analysis
[125]. However, given the complexity of the model and the failure to take into account post-transplantation risk factors, it seems difficult to use in clinical practice. Sharma’s team suggests calculating a Renal Risk Index (RRI) to predict the risk of end-stage renal disease following liver transplantation
[96]. It estimates the cumulative incidence of ESRD at 1, 3 and 5 years post-liver transplantation by taking into account age; BMI; ethnicity; UNOS status; the presence of TIPS; and laboratory values, such as serum creatinine, bilirubin, albumin and natremia. Finally, the AKI score, which is more recent, categorizes risks into three distinct groups based on five factors: the BMI of the donor and the recipient, the time of warm ischemia, the characteristic of the graft (deceased donors) and the need for fresh frozen plasma during the procedure
[141][148]. However, these scores are still rarely used on a daily basis and still need to be validated in routine practice.
5. Prevention of Post-Liver Transplantation Kidney Failure
The prevention of CKD after liver transplantation should be considered a priority due to the considerable impact on patient morbidity and mortality. Therefore, the early management of modifiable risk factors in these patients is essential.
5.1. Pre-Transplantation Prevention
Preventing pre-transplantation AKI is a major factor in the prevention of post-transplantation kidney failure. This implies limiting the use of nephrotoxic treatments (NSAIDs, iodinated contrast products and aminoglycosides), compensating ascites and treating ascetic fluid infections (AFI) by albumin infusion, the early treatment of all infections, the implementation of antibiotic prophylaxis for AFI and the early management of episodes of hemodynamic instability or hepato-renal syndrome. In this regard, the O’Leary team proposes an algorithm for the management of AKI in patients with cirrhosis regarding the mechanisms of the AKI (pre-renal, renal and post-renal) and the AKI evolution at 48 h after treatment
[142][149].
5.2. Post-Transplantation Prevention
5.2.1. Control of Cardiovascular Risk Factors
Cardiovascular risk factors are very frequent in liver transplant patients. First of all, cirrhosis is responsible for hypermetabolism associated with insulin resistance. Second, immunosuppressive drugs, such as tacrolimus or corticosteroids, cause high blood pressure, dyslipidemia and diabetes. These complications are associated with a decline in kidney function after liver transplantation. Consequently, strict control is required before and after transplantation to minimize the impact on kidney function
[20]. Therefore, immunosuppression without corticosteroids is preferable. Two meta-analyses have shown that an immunosuppressive regimen without corticosteroid therapy has no impact on renal function, patient survival or short- and long-term liver transplant survival, but can limit the occurrence of diabetes or dyslipidemia
[143][144][150,151]. Finally, hyperuricemia, a frequent complication after organ transplantation, seems to be correlated with the development of CKD in liver transplant patients. Similarly, treatment with allopurinol leads to a significant decrease in creatinine level after liver transplantation
[88][145][88,152].
5.2.2. Optimization of Immunosuppressive Treatment
Calcineurin inhibitors (CNIs) (cyclosporine and tacrolimus) are the cornerstone of immunosuppressive treatment in organ transplantation, despite their nephrotoxicity. This chronic nephrotoxicity is characterized by arteriolar hyalinosis, which leads to a variety of tubulointerstitial and glomerular lesions with an essentially hemodynamic and ischemic mechanism. Acute rejection is less likely with liver transplants than other organ transplants. Therefore, the current dynamic is to decrease exposure to CNIs to minimize kidney toxicity (9). At present, the available strategies are (1) promoting the use of tacrolimus over cyclosporine; (2) reducing CNIs by adding mycophenolic acid (MPA) or mTor (mammalian target of rapamycin) inhibitor; (3) delaying the introduction of CNIs after induction therapy (basiliximab or antilymphocyte serum); (4) complete weaning from CNIs by early conversion to mTor inhibitors or antimetabolites.
6. The Indication for Liver–Kidney Transplantation
With the increase in the incidence of kidney failure in patients awaiting liver transplantation and with the use of the MELD score, the indication for combined liver–kidney transplantation has been on the rise since 2002
[146][186]. However, it is often difficult to decide whether a dual transplantation is necessary as the assessment of the reversibility of kidney damage is complex, as is the risk of post-transplantation ESRD. Hmoud et al. showed that among patients listed for combined transplantation but who finally received liver transplantation alone, one-third exhibited recovered kidney function with a GFR of more than 60 mL/min/1.73 m
2 [147][187]. Similarly, the survival benefit of a combined liver–kidney transplant is currently uncertain. Some studies have shown an advantage of combined transplantation in terms of survival, while others have shown no superiority
[148][149][150][188,189,190]. However, most of these are observational monocentric studies conducted in a small number of subjects. In a study of 5609 patients who received combined or isolated liver transplant, Sharma et al. showed that the 5-year survival in patients on dialysis before transplantation was similar in both groups. On the other hand, there is a slight improvement in survival in favor of combined transplantation in patients not on dialysis at the time of transplantation (+3.7 months concentrated mainly in the first year). This is partly due to a better quality of kidney and liver transplants in combined transplants
[151][191]. However, renal transplantation performed in a certain period of time after liver transplantation or staggered for 24–48 h (kidney on perfusion machine) appears to cause less morbidity and to have a protective effect on the renal transplant. This is partly explained by less exposure to SIRS in the post-liver transplantation period, which is responsible for early and late renal dysfunction
[152][192]. A recent study reports better survival for patients who receive a liver transplant and then a kidney transplant within 3 years compared to patients who receive a liver and a kidney transplant simultaneously
[153][193]. However, one of the advantages of combined transplantation remains immunological with a reduced risk of rejection in patients with pre-formed DSAs that allow the long-term preservation of kidney function
[154][194]. In order to limit the indications for dual transplantation and to standardize the practices of each center, in 2017, an expert committee published the latest recommendations for combined liver–kidney transplantation in patients with cirrhosis who are on the waiting list (
Table 1)
[69]. It can be noted that the indications for dual transplantation in the case of CKD are relatively homogeneous over time, in contrast to the indications for combined transplantation for AKI, which remain more heterogeneous
[155][195].
Table 1. Current indications for combined liver–kidney transplantation (OPTN 2017 recommendations)
[69].
|
Indications for a Combined Liver–Kidney Transplantation
|
|
Patients with AKI associated with:
-
▪
-
A need for dialysis ≥6 weeks;
-
▪
-
GFR estimated according to MDRD-6 ≤ 25 mL/min for more than 6 weeks.
|
|
Patients with CKD (eGFR < 60 mL/min for at least 3 months) associated with:
-
▪
-
Recent worsening of GFR ≤ 30 mL/min estimated according to MDRD6;
-
▪
-
A need for hemodialysis;
-
▪
-
A metabolic disease (hyperoxaluria, methylmalonic aciduria, atypical HUS with factor H or I mutation or hereditary amyloidosis).
|
AKI: acute kidney injury; GFR: glomerular filtration rate; MDRD: modification of diet in renal disease; CKD: chronic kidney disease; HUS: hemolytic uremic syndrome.